Contributions
Abstract: PB1570
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Although many patients (pts) with follicular lymphoma (FL) respond to first-line therapy, most pts have a history of successive relapses and response duration shortens after each relapse; therefore, FL is considered incurable and an area of considerable unmet need. Loncastuximab tesirine (Lonca) is an antibody-drug conjugate comprising a humanized anti-CD19 monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer (PBD) toxin. Single-agent Lonca demonstrated antitumor activity and a manageable safety profile in pts with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma, including FL, in a Phase 1 clinical trial (ADCT-402-101), and in heavily pretreated pts with R/R diffuse large B-cell lymphoma in a Phase 2 trial (ADCT-402-201). Idelalisib is an inhibitor of phosphatidylinositol 3-kinase, approved for pts with relapsed FL who had ≥2 prior systemic therapies.
Aims
The primary objective of this trial (NCT04699461) is to evaluate the efficacy of single-agent Lonca compared with idelalisib in R/R FL.
Methods
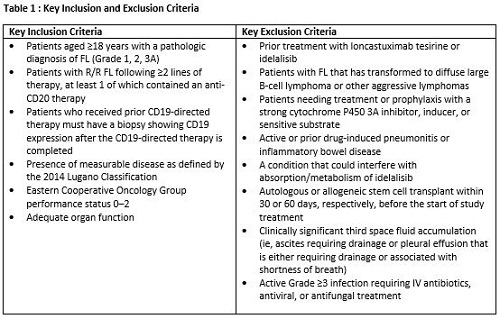
This is a prospective, randomized, two-arm, multicenter, open-label, Phase 2 study of Lonca versus idelalisib in pts with R/R FL. Key inclusion and exclusion criteria for the study are presented in Table 1. Informed consent will be obtained. Eligible pts will be randomized 2:1 to Lonca (intravenous infusion; 150 µg/kg every 3 weeks [Q3W] for 2 cycles then 75 µg/kg Q3W for subsequent cycles) or idelalisib (oral; 150 mg twice daily). Randomization will be stratified based on the time since last systemic therapy (ie, ≤2 years versus >2 years). The follow-up period will be up to 3 years after the end of treatment. Pts can continue treatment until disease progression, unacceptable toxicity, or other discontinuation criteria.
The primary endpoint is complete response rate (CRR) according to the 2014 Lugano Classification as determined by central review. CRR will be tested for the intent-to-treat population between the two treatment arms using the Cochran–Mantel–Haenszel method adjusted for stratification factors from the randomization list. The study is 99% powered for the primary efficacy endpoint, assuming a 55% CRR to Lonca and a 15% CRR to idelalisib, and the sample size for enrollment (approximately 150 pts) allows for a robust safety data set. Secondary endpoints include overall response rate (key secondary endpoint); progression-free survival; overall survival; duration of response; incidence and severity of adverse events (AEs) and serious AEs; change from baseline in laboratory values, vital signs, 12-lead electrocardiogram, and Eastern Cooperative Oncology Group performance status; concentration and pharmacokinetic parameters of Lonca total antibody, PBD-conjugated antibody, and unconjugated warhead (SG3199); anti-drug antibody titers; and patient-reported outcome (PRO) measures. PRO measures will include change from baseline in PROs, as measured by EuroQol–5 Dimensions–5 Levels and Functional Assessment of Cancer Therapy-Lymphoma, and the occurrence, severity, and interference of specific symptomatic AEs selected from the PROs version of the Common Terminology Criteria for AEs.
The trial is anticipated to open for recruitment in Quarter 1, 2021.
This study is sponsored by ADC Therapeutics SA.
Results
N/A
Conclusion
N/A
Keyword(s): Follicular lymphoma, Immunotherapy, Phase II, Randomized
Abstract: PB1570
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Although many patients (pts) with follicular lymphoma (FL) respond to first-line therapy, most pts have a history of successive relapses and response duration shortens after each relapse; therefore, FL is considered incurable and an area of considerable unmet need. Loncastuximab tesirine (Lonca) is an antibody-drug conjugate comprising a humanized anti-CD19 monoclonal antibody conjugated to a pyrrolobenzodiazepine dimer (PBD) toxin. Single-agent Lonca demonstrated antitumor activity and a manageable safety profile in pts with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma, including FL, in a Phase 1 clinical trial (ADCT-402-101), and in heavily pretreated pts with R/R diffuse large B-cell lymphoma in a Phase 2 trial (ADCT-402-201). Idelalisib is an inhibitor of phosphatidylinositol 3-kinase, approved for pts with relapsed FL who had ≥2 prior systemic therapies.
Aims
The primary objective of this trial (NCT04699461) is to evaluate the efficacy of single-agent Lonca compared with idelalisib in R/R FL.
Methods
This is a prospective, randomized, two-arm, multicenter, open-label, Phase 2 study of Lonca versus idelalisib in pts with R/R FL. Key inclusion and exclusion criteria for the study are presented in Table 1. Informed consent will be obtained. Eligible pts will be randomized 2:1 to Lonca (intravenous infusion; 150 µg/kg every 3 weeks [Q3W] for 2 cycles then 75 µg/kg Q3W for subsequent cycles) or idelalisib (oral; 150 mg twice daily). Randomization will be stratified based on the time since last systemic therapy (ie, ≤2 years versus >2 years). The follow-up period will be up to 3 years after the end of treatment. Pts can continue treatment until disease progression, unacceptable toxicity, or other discontinuation criteria.
The primary endpoint is complete response rate (CRR) according to the 2014 Lugano Classification as determined by central review. CRR will be tested for the intent-to-treat population between the two treatment arms using the Cochran–Mantel–Haenszel method adjusted for stratification factors from the randomization list. The study is 99% powered for the primary efficacy endpoint, assuming a 55% CRR to Lonca and a 15% CRR to idelalisib, and the sample size for enrollment (approximately 150 pts) allows for a robust safety data set. Secondary endpoints include overall response rate (key secondary endpoint); progression-free survival; overall survival; duration of response; incidence and severity of adverse events (AEs) and serious AEs; change from baseline in laboratory values, vital signs, 12-lead electrocardiogram, and Eastern Cooperative Oncology Group performance status; concentration and pharmacokinetic parameters of Lonca total antibody, PBD-conjugated antibody, and unconjugated warhead (SG3199); anti-drug antibody titers; and patient-reported outcome (PRO) measures. PRO measures will include change from baseline in PROs, as measured by EuroQol–5 Dimensions–5 Levels and Functional Assessment of Cancer Therapy-Lymphoma, and the occurrence, severity, and interference of specific symptomatic AEs selected from the PROs version of the Common Terminology Criteria for AEs.
The trial is anticipated to open for recruitment in Quarter 1, 2021.
This study is sponsored by ADC Therapeutics SA.
Results
N/A
Conclusion
N/A
Keyword(s): Follicular lymphoma, Immunotherapy, Phase II, Randomized