
Contributions
Abstract: PB1565
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Despite currently available therapies, FL is characterized by recurrent relapses, with increasing refractoriness and decreasing duration of response to subsequent lines of therapy. Mosunetuzumab is a full length, IgG1 CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab monotherapy elicited high and consistent complete response (CR) and overall response rates (ORR) across high-risk FL subsets in an ongoing Phase I/Ib study (N=62; CR rate: 51.6%; ORR: 67.7%; NCT02500407; Assouline, et al. ASH 2020). Lenalidomide is a potent immunomodulatory agent that has been shown to enhance T‑cell and natural killer‑cell activity. As such, mosunetuzumab and lenalidomide have potential to exhibit synergistic activity. The combination of M-Len may afford further clinical benefit, providing a new treatment option for patients with R/R FL. Here we present details of a trial in progress assessing M-Len.
Aims
To evaluate the efficacy and safety of M-Len versus R-Len in patients with R/R FL.
Methods
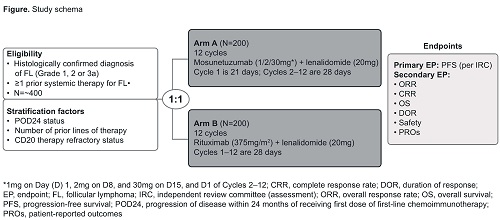
GO42909 (NCT04712097) is a Phase III, open-label, multicenter, randomized controlled trial in patients with R/R FL. Eligible patients must have CD20-positive FL (excluding grade 3b and transformed FL) with an Eastern Cooperative Oncology Group Performance status ≤2, patients must also have received at least one prior systemic therapy, and require treatment according to Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria (Solal-Céligny, et al. J Clin Oncol 1998). Patients are randomized 1:1 to receive either M-Len or R-Len; randomization is stratified by progression of disease within 24 months of receiving the first dose of first-line immunochemotherapy, number of prior therapies, and CD20-refractoriness. Patients in the M-Len cohort receive mosunetuzumab intravenously (IV) as step-up doses in Cycle 1 (21-day cycle): 1mg on Day (D)1, 2mg on D8, and 30mg on D15, and D1 of Cycles 2–12 (28-day cycles). Lenalidomide (20mg) is administered orally, once daily on D1–21 of Cycles 2–12. Patients in the R-Len cohort receive 375mg/m2 rituximab (IV) on D1, 8, 15 and 22 of Cycle 1, and on D1 of Cycles 3, 5, 7, 9 and 11 (28-day cycles); lenalidomide is administered once a day on D1–21 of Cycles 1–12 (Figure). The primary efficacy endpoint is progression-free survival as determined by an independent review committee using the 2014 Lugano response criteria (Cheson, et al. J Clin Oncol 2014). Key secondary efficacy endpoints include ORR, CR rate, and overall survival. Safety endpoints comprise incidence and severity of adverse events (NCI CTCAE v5.0) including cytokine release syndrome (ASTCT criteria; Lee, et al. Biol Blood Marrow Transplant 2019), change from baseline in targeted vital signs and clinical laboratory test results, and tolerability; an independent data monitoring committee will evaluate safety data periodically during the study. In addition, pharmacokinetic and biomarker endpoints will be explored.
Results
The study start date is planned for 2021, with an anticipated enrollment of around 400 patients from approximately 16 countries and at least 150 sites globally.
Conclusion
GO42909 is an ongoing study of M-Len versus R-Len in patients with R/R FL. Further study details will be presented.
Keyword(s): Follicular lymphoma, Phase III, Refractory, Relapsed lymphoma
Abstract: PB1565
Type: Publication Only
Session title: Indolent and mantle-cell non-Hodgkin lymphoma - Clinical
Background
Despite currently available therapies, FL is characterized by recurrent relapses, with increasing refractoriness and decreasing duration of response to subsequent lines of therapy. Mosunetuzumab is a full length, IgG1 CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab monotherapy elicited high and consistent complete response (CR) and overall response rates (ORR) across high-risk FL subsets in an ongoing Phase I/Ib study (N=62; CR rate: 51.6%; ORR: 67.7%; NCT02500407; Assouline, et al. ASH 2020). Lenalidomide is a potent immunomodulatory agent that has been shown to enhance T‑cell and natural killer‑cell activity. As such, mosunetuzumab and lenalidomide have potential to exhibit synergistic activity. The combination of M-Len may afford further clinical benefit, providing a new treatment option for patients with R/R FL. Here we present details of a trial in progress assessing M-Len.
Aims
To evaluate the efficacy and safety of M-Len versus R-Len in patients with R/R FL.
Methods
GO42909 (NCT04712097) is a Phase III, open-label, multicenter, randomized controlled trial in patients with R/R FL. Eligible patients must have CD20-positive FL (excluding grade 3b and transformed FL) with an Eastern Cooperative Oncology Group Performance status ≤2, patients must also have received at least one prior systemic therapy, and require treatment according to Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria (Solal-Céligny, et al. J Clin Oncol 1998). Patients are randomized 1:1 to receive either M-Len or R-Len; randomization is stratified by progression of disease within 24 months of receiving the first dose of first-line immunochemotherapy, number of prior therapies, and CD20-refractoriness. Patients in the M-Len cohort receive mosunetuzumab intravenously (IV) as step-up doses in Cycle 1 (21-day cycle): 1mg on Day (D)1, 2mg on D8, and 30mg on D15, and D1 of Cycles 2–12 (28-day cycles). Lenalidomide (20mg) is administered orally, once daily on D1–21 of Cycles 2–12. Patients in the R-Len cohort receive 375mg/m2 rituximab (IV) on D1, 8, 15 and 22 of Cycle 1, and on D1 of Cycles 3, 5, 7, 9 and 11 (28-day cycles); lenalidomide is administered once a day on D1–21 of Cycles 1–12 (Figure). The primary efficacy endpoint is progression-free survival as determined by an independent review committee using the 2014 Lugano response criteria (Cheson, et al. J Clin Oncol 2014). Key secondary efficacy endpoints include ORR, CR rate, and overall survival. Safety endpoints comprise incidence and severity of adverse events (NCI CTCAE v5.0) including cytokine release syndrome (ASTCT criteria; Lee, et al. Biol Blood Marrow Transplant 2019), change from baseline in targeted vital signs and clinical laboratory test results, and tolerability; an independent data monitoring committee will evaluate safety data periodically during the study. In addition, pharmacokinetic and biomarker endpoints will be explored.
Results
The study start date is planned for 2021, with an anticipated enrollment of around 400 patients from approximately 16 countries and at least 150 sites globally.

Conclusion
GO42909 is an ongoing study of M-Len versus R-Len in patients with R/R FL. Further study details will be presented.
Keyword(s): Follicular lymphoma, Phase III, Refractory, Relapsed lymphoma


