
Contributions
Abstract: PB1555
Type: Publication Only
Session title: Hodgkin lymphoma - Clinical
Background
Classical Hodgkin Lymphoma (cHL) constitutes 95% of all reported cases of HL and is often cured with chemotherapy. Approximately 10% to 20% of the patients progress to relapsed/refractory cHL (RRHL), requiring intense treatment with chemotherapy and hematopoietic stem cell transplantation (SCT). Differences in clinicopathological characteristics based on geographical and socioeconomic status are reported previously.
Aims
In this subgroup analysis of the B-CD30+ HOdgkin Lymphoma International Multicenter Retrospective Study of Treatment PractIces and OutComes (B-HOLISTIC) (NCT03327571) study, we report the treatment pathways and outcomes in patients from China with cHL and RRHL.
Methods
This retrospective, multicenter, observational study retrieved data from the medical records of patients (≥18 years) with a diagnosis of stage IIb-IV cHL between January 1, 2010 and December 31, 2013 who have received frontline treatment with chemotherapy with or without radiotherapy (Group 1) and of patients with a diagnosis of RRHL (Group 2). Patients who progressed from cHL to RRHL during the study period were included in both the groups. The primary end point was progression-free survival (PFS) in patients with RRHL and the secondary end points were overall survival (OS), best clinical response, and incidences of adverse events (AEs). Survival analysis (OS and PFS) was performed using Kaplan Meier method, and the differences in baseline factors were adjusted with multivariable analysis.
Results
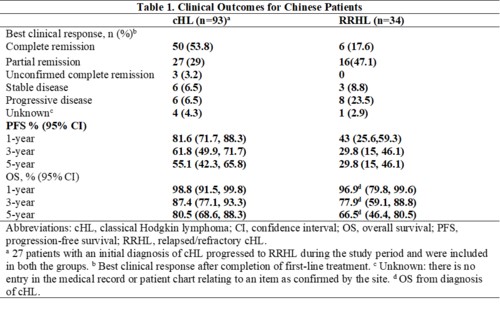
In all, 93 and 34 Chinese patients were enrolled in the cHL and RRHL groups, respectively (27 patients in the cHL group progressed to RRHL and were included in both the groups). The median age (range) of patients at diagnosis in the cHL group was 33 (18-88) years, and 62/93 (66.7%) patients were males. The distribution of clinical stage (Ann Arbor staging) at cHL diagnosis was as follows: stage II, 18/93 (19.4%); stage III, 43/93 (46.2%); stage IV, 32/93 (34.4%). All patients in the cHL group received first-line chemotherapy, with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD; 78/93 [83.9%]) and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone (BEACOPP; 17/93 [18.3%]) being the most prevalent chemotherapeutic regimen. A total of 12/93 (12.9%) patients in the cHL group also received consolidation radiotherapy. The median age (range) at diagnosis for patients in the RRHL group was 34 (20-73) years, and 20/34 (58.8%) were males. At RRHL diagnosis, intensive second-line chemotherapy was used in 31/34 (91.2%) patients: the common regimens used in second line were BEACOPP (5/34 [16.1%]), cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) (5/34 [16.1%]), and ABVD (4/34 [12.9%]). Of the 34 patients with RRHL, 28 (82.4%) were eligible for hematopoietic SCT at relapse/refractory diagnosis; Among the 34 patients, 13 patients (38.2%) patients underwent autologous SCT and 1 patient underwent allogenic SCT. Median PFS (95% confidence interval) for the RRHL group was 9.53 (3.84-20.43) months, with estimated 1-, 3-, and 5-year PFS rates of 43%, 29.8%, and 29.8%, respectively (Table 1). Median OS was not reached for both the groups. AEs were reported by 67/93 (72%) patients with cHL and 31/34 (91.2%) patients with RRHL.
Conclusion
Subgroup analyses of Chinese patients from the B-HOLISTIC study reveal that the PFS remains low in patients with RRHL in real-world clinical practice in China. This emphasizes the unmet need for novel targeted therapies to improve survival in patients with RRHL.
Keyword(s): Hodgkin's lymphoma, Targeted therapy
Abstract: PB1555
Type: Publication Only
Session title: Hodgkin lymphoma - Clinical
Background
Classical Hodgkin Lymphoma (cHL) constitutes 95% of all reported cases of HL and is often cured with chemotherapy. Approximately 10% to 20% of the patients progress to relapsed/refractory cHL (RRHL), requiring intense treatment with chemotherapy and hematopoietic stem cell transplantation (SCT). Differences in clinicopathological characteristics based on geographical and socioeconomic status are reported previously.
Aims
In this subgroup analysis of the B-CD30+ HOdgkin Lymphoma International Multicenter Retrospective Study of Treatment PractIces and OutComes (B-HOLISTIC) (NCT03327571) study, we report the treatment pathways and outcomes in patients from China with cHL and RRHL.
Methods
This retrospective, multicenter, observational study retrieved data from the medical records of patients (≥18 years) with a diagnosis of stage IIb-IV cHL between January 1, 2010 and December 31, 2013 who have received frontline treatment with chemotherapy with or without radiotherapy (Group 1) and of patients with a diagnosis of RRHL (Group 2). Patients who progressed from cHL to RRHL during the study period were included in both the groups. The primary end point was progression-free survival (PFS) in patients with RRHL and the secondary end points were overall survival (OS), best clinical response, and incidences of adverse events (AEs). Survival analysis (OS and PFS) was performed using Kaplan Meier method, and the differences in baseline factors were adjusted with multivariable analysis.
Results
In all, 93 and 34 Chinese patients were enrolled in the cHL and RRHL groups, respectively (27 patients in the cHL group progressed to RRHL and were included in both the groups). The median age (range) of patients at diagnosis in the cHL group was 33 (18-88) years, and 62/93 (66.7%) patients were males. The distribution of clinical stage (Ann Arbor staging) at cHL diagnosis was as follows: stage II, 18/93 (19.4%); stage III, 43/93 (46.2%); stage IV, 32/93 (34.4%). All patients in the cHL group received first-line chemotherapy, with doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD; 78/93 [83.9%]) and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone (BEACOPP; 17/93 [18.3%]) being the most prevalent chemotherapeutic regimen. A total of 12/93 (12.9%) patients in the cHL group also received consolidation radiotherapy. The median age (range) at diagnosis for patients in the RRHL group was 34 (20-73) years, and 20/34 (58.8%) were males. At RRHL diagnosis, intensive second-line chemotherapy was used in 31/34 (91.2%) patients: the common regimens used in second line were BEACOPP (5/34 [16.1%]), cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) (5/34 [16.1%]), and ABVD (4/34 [12.9%]). Of the 34 patients with RRHL, 28 (82.4%) were eligible for hematopoietic SCT at relapse/refractory diagnosis; Among the 34 patients, 13 patients (38.2%) patients underwent autologous SCT and 1 patient underwent allogenic SCT. Median PFS (95% confidence interval) for the RRHL group was 9.53 (3.84-20.43) months, with estimated 1-, 3-, and 5-year PFS rates of 43%, 29.8%, and 29.8%, respectively (Table 1). Median OS was not reached for both the groups. AEs were reported by 67/93 (72%) patients with cHL and 31/34 (91.2%) patients with RRHL.

Conclusion
Subgroup analyses of Chinese patients from the B-HOLISTIC study reveal that the PFS remains low in patients with RRHL in real-world clinical practice in China. This emphasizes the unmet need for novel targeted therapies to improve survival in patients with RRHL.
Keyword(s): Hodgkin's lymphoma, Targeted therapy


