
Contributions
Abstract: PB1547
Type: Publication Only
Session title: Hematopoiesis, stem cells and microenvironment
Background
Anemia is a universal complication of End Stage Renal Disease (ESRD). Erythropoiesis-stimulating agents (ESAs) have fundamentally changed the treatment of anemia of ESRD, greatly decreasing transfusion dependence. However, ESA-resistant anemia is not well studied and a difficult condition to manage. Among the most frequent causes of ESA-resistant anemia is secondary hyperparathyroidism (SHP), which is associated with bone marrow remodeling and bone marrow fibrosis. Treatment goals of SHP are centered on goal calcium, phosphorus and parathyroid hormone (PTH) levels determined largely by consensus, and not pathophysiology including hematologic effects.
Aims
Here we describe a patient with ESRD, SHP with reversible bone marrow changes and transfusion-dependent anemia, as well as descriptive findings of bone marrow changes in patients with anemia and ESRD in our center.
Methods
Using the EMR, the patient's data including demographics, progress notes, laboratoy values, and bone marrow findings were extracted. Subsequently, the EMR was interrogated to reveal all patients with anemia and ESRD, who had a bone marrow biopsy at out center. Data including demographics, laboratory values, bone marrow findings, and medications were extracted. Data was presented descriptively.
Results
A 67-year-old male with ESRD secondary to hypertension and type 2 diabetes was referred to our Hematology clinic for anemia of ESRD. He was receiving epoeitin 20,000 three times weekly. He was transfusion-dependent to maintain a hemoglobin of 6.3 g/dL, with a concomitant PTH of 400.5 pg/mL. Calcium and phosphorus were normal. Bone marrow biopsy revealed focal areas of bone marrow remodeling and reticulin fibrosis. Fluorescence in situ hybridization studies for myelodysplastic syndrome were negative, as were thyroid studies, serum protein electrophoresis (SPEP), and free light chain ratio. In conjunction with his Nephrologist, treatment of his SHP was optimized. His initial regimen was calcitriol 0.25 mcg once daily and calcium acetate 667 mg three times daily. Subsequently, cinacalcet 30 mg twice daily was added and calcium acetate was replaced with sevelamer 1600 mg three times daily. Following these changes, his hemoglobin remained stable in the range of 8-9 g/dL. He became transfusion independent, despite only a modest reduction in PTH to 311.2 pg/mL. Repeat bone marrow biopsy revealed no bone marrow remodeling or reticulin fibrosis.
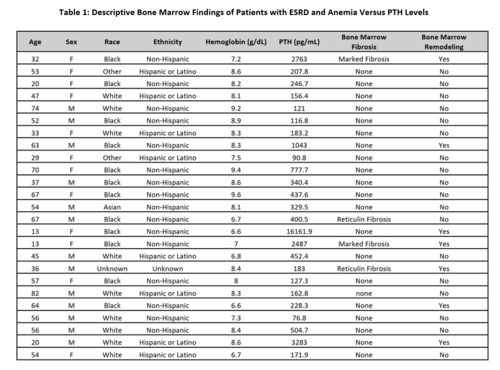
Bone marrow findings for all patients in our center with ESRD and anemia are summarized in Table 1. The range of PTH for patients with bony remodeling was 183-16161.9 pg/mL versus 90.8-3283 pg/mL for those without bony remodeling. The range of PTH for patients with fibrotic changes was 183-2487 pg/mL versus 90.8-16161.9 pg/mL for those without fibrotic changes.
Conclusion
SHP is an increasingly defined cause of ESA-resistant anemia in ESRD. Here we describe a patient with modestly elevated PTH and reversibility of his transfusion-dependent anemia and bone marrow changes following optimized management of his SHP. The degree of reversible ESA-resistant anemia and transfusion dependence in the setting of a PTH elevation to 400.5 in this case is surprising. The great variability in PTH levels of both affected and unaffected patients (i.e. bone marrow changes) presented here indicates the relationship between SHP and anemia is complex. A single level for the whole dialysis population is not appropriate. It may be reasonable to attempt a trial of more aggressive management of SHP in patients with difficult to manage ESA-resistant anemia and only mild-moderate PTH elevation.
Keyword(s): Anemia, Renal failure, Transfusion
Abstract: PB1547
Type: Publication Only
Session title: Hematopoiesis, stem cells and microenvironment
Background
Anemia is a universal complication of End Stage Renal Disease (ESRD). Erythropoiesis-stimulating agents (ESAs) have fundamentally changed the treatment of anemia of ESRD, greatly decreasing transfusion dependence. However, ESA-resistant anemia is not well studied and a difficult condition to manage. Among the most frequent causes of ESA-resistant anemia is secondary hyperparathyroidism (SHP), which is associated with bone marrow remodeling and bone marrow fibrosis. Treatment goals of SHP are centered on goal calcium, phosphorus and parathyroid hormone (PTH) levels determined largely by consensus, and not pathophysiology including hematologic effects.
Aims
Here we describe a patient with ESRD, SHP with reversible bone marrow changes and transfusion-dependent anemia, as well as descriptive findings of bone marrow changes in patients with anemia and ESRD in our center.
Methods
Using the EMR, the patient's data including demographics, progress notes, laboratoy values, and bone marrow findings were extracted. Subsequently, the EMR was interrogated to reveal all patients with anemia and ESRD, who had a bone marrow biopsy at out center. Data including demographics, laboratory values, bone marrow findings, and medications were extracted. Data was presented descriptively.
Results
A 67-year-old male with ESRD secondary to hypertension and type 2 diabetes was referred to our Hematology clinic for anemia of ESRD. He was receiving epoeitin 20,000 three times weekly. He was transfusion-dependent to maintain a hemoglobin of 6.3 g/dL, with a concomitant PTH of 400.5 pg/mL. Calcium and phosphorus were normal. Bone marrow biopsy revealed focal areas of bone marrow remodeling and reticulin fibrosis. Fluorescence in situ hybridization studies for myelodysplastic syndrome were negative, as were thyroid studies, serum protein electrophoresis (SPEP), and free light chain ratio. In conjunction with his Nephrologist, treatment of his SHP was optimized. His initial regimen was calcitriol 0.25 mcg once daily and calcium acetate 667 mg three times daily. Subsequently, cinacalcet 30 mg twice daily was added and calcium acetate was replaced with sevelamer 1600 mg three times daily. Following these changes, his hemoglobin remained stable in the range of 8-9 g/dL. He became transfusion independent, despite only a modest reduction in PTH to 311.2 pg/mL. Repeat bone marrow biopsy revealed no bone marrow remodeling or reticulin fibrosis.
Bone marrow findings for all patients in our center with ESRD and anemia are summarized in Table 1. The range of PTH for patients with bony remodeling was 183-16161.9 pg/mL versus 90.8-3283 pg/mL for those without bony remodeling. The range of PTH for patients with fibrotic changes was 183-2487 pg/mL versus 90.8-16161.9 pg/mL for those without fibrotic changes.

Conclusion
SHP is an increasingly defined cause of ESA-resistant anemia in ESRD. Here we describe a patient with modestly elevated PTH and reversibility of his transfusion-dependent anemia and bone marrow changes following optimized management of his SHP. The degree of reversible ESA-resistant anemia and transfusion dependence in the setting of a PTH elevation to 400.5 in this case is surprising. The great variability in PTH levels of both affected and unaffected patients (i.e. bone marrow changes) presented here indicates the relationship between SHP and anemia is complex. A single level for the whole dialysis population is not appropriate. It may be reasonable to attempt a trial of more aggressive management of SHP in patients with difficult to manage ESA-resistant anemia and only mild-moderate PTH elevation.
Keyword(s): Anemia, Renal failure, Transfusion


