
Contributions
Abstract: PB1546
Type: Publication Only
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Genetically engineered CAR T cells represent a breakthrough therapeutic innovation for lymphoma patients in second or more relapse. Despite clinical trials, there is a lack of data, especially about drug use and Complementary and Alternative Medicines (CAM). Such data are necessary to optimize patient care and safety and to improve knowledge about this recent population.
Aims
We performed a prospective real-world study to evaluate drug use and CAM practice in lymphoma patients treated with axi-cel, tisa-cel and brexu-cel.
Methods
Data from patients who received CAR T cells between May and November 2020, outside of clinical trials, were prospectively collected and analyzed. Drug use and CAM were collected during an interview between a hospital pharmacist and the patient the day before lymphodepletion chemotherapy and at month 3 after CAR T cell infusion.
Results
25 successive patients were infused among them 17 patients with DLBCL and one with mantle cell lymphoma were analysed. Patients were treated by axi-cel (11), tisa-cel (7) and brexu-cel (1). Six of the 18 patients were excluded from the study in the first 3 months (1 death, 3 progressions, 2 refusals).
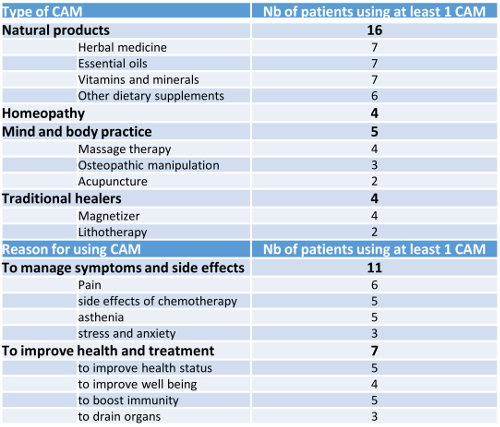
The male/female sex ratio was 1.25 (10/18), and mean age at infusion 59 years [range, 20-77 years], with 100% of the patients with ECOG performance status ≤ 1. Median number of prior therapies was 2 [range, 2-4]. Median Charlson score was 2 [range, 0-5]. Half of the patients were unemployed or retired (n=9) at diagnosis. Some patients were polymedicated (n=9), using ≥ 5 daily prescribed drugs; the most frequent classes were anti-infectious (n=13), anti-ulcer drugs (n=10), cardiovascular drugs (n=4) as well as thromboprophylaxis agents (n=3). 16 patients used analgesics (prescription or self-medication). Psychotropic drugs were taken by 5 patients (28%) to manage stress, anxiety, sleep disorders or depression; 16/18 patients used at least one CAM with a mean of 3.3 [range, 1-12] (Table). No changes of drug use and CAM were observed through the first 3 months following the infusion of CAR T.
Conclusion
This study provides one of the first datasets on drug use and CAM in lymphoma patients treated by CAR T cells in real-life. Despite CAR T cells bring an innovative and expensive strategy, lymphoma patients continue using CAM to manage health disorders and to boost immunity as other cancer patients treated with chemotherapy. Such results should led us to investigate patients’ representations about innovative anticancer drugs.
Keyword(s): CAR-T, Lymphoma
Abstract: PB1546
Type: Publication Only
Session title: Gene therapy, cellular immunotherapy and vaccination - Clinical
Background
Genetically engineered CAR T cells represent a breakthrough therapeutic innovation for lymphoma patients in second or more relapse. Despite clinical trials, there is a lack of data, especially about drug use and Complementary and Alternative Medicines (CAM). Such data are necessary to optimize patient care and safety and to improve knowledge about this recent population.
Aims
We performed a prospective real-world study to evaluate drug use and CAM practice in lymphoma patients treated with axi-cel, tisa-cel and brexu-cel.
Methods
Data from patients who received CAR T cells between May and November 2020, outside of clinical trials, were prospectively collected and analyzed. Drug use and CAM were collected during an interview between a hospital pharmacist and the patient the day before lymphodepletion chemotherapy and at month 3 after CAR T cell infusion.
Results
25 successive patients were infused among them 17 patients with DLBCL and one with mantle cell lymphoma were analysed. Patients were treated by axi-cel (11), tisa-cel (7) and brexu-cel (1). Six of the 18 patients were excluded from the study in the first 3 months (1 death, 3 progressions, 2 refusals).
The male/female sex ratio was 1.25 (10/18), and mean age at infusion 59 years [range, 20-77 years], with 100% of the patients with ECOG performance status ≤ 1. Median number of prior therapies was 2 [range, 2-4]. Median Charlson score was 2 [range, 0-5]. Half of the patients were unemployed or retired (n=9) at diagnosis. Some patients were polymedicated (n=9), using ≥ 5 daily prescribed drugs; the most frequent classes were anti-infectious (n=13), anti-ulcer drugs (n=10), cardiovascular drugs (n=4) as well as thromboprophylaxis agents (n=3). 16 patients used analgesics (prescription or self-medication). Psychotropic drugs were taken by 5 patients (28%) to manage stress, anxiety, sleep disorders or depression; 16/18 patients used at least one CAM with a mean of 3.3 [range, 1-12] (Table). No changes of drug use and CAM were observed through the first 3 months following the infusion of CAR T.

Conclusion
This study provides one of the first datasets on drug use and CAM in lymphoma patients treated by CAR T cells in real-life. Despite CAR T cells bring an innovative and expensive strategy, lymphoma patients continue using CAM to manage health disorders and to boost immunity as other cancer patients treated with chemotherapy. Such results should led us to investigate patients’ representations about innovative anticancer drugs.
Keyword(s): CAR-T, Lymphoma


