
Contributions
Abstract: PB1518
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
Long-term survival in chronic myeloid leukemia (CML) has been achieved with the advent of the BCR-ABL tyrosine kinase inhibitors (TKIs), which represent the standard treatment of this disease nowadays. However, almost 20 % of LMC patients present TKIs intolerance, and finding the right dose while maintaining an optimal response, without therapy-related adverse events, is challenging.
Aims
The aim of our study was to retrospectively analyze the impact of low dose TKIs on the deep molecular response (MR) in the group of patients who required a dose reduction due to adverse effects or in order to prevent long-term toxicities, in comparison with those who maintained standard dose.
Methods
We analyzed 82 patients diagnosed with chronic phase of CML from January 2000 to December 2020, who started treatment with TKIs.
Results
Among the population of study, 47 (58,02%) were male and 35 (42,6%) female, with a median age of 50 years (range 13-87ys), at the moment of diagnosis.
Standard dose of Imatinib (400mg/day) was the first line TKI in 63 (76,8%) patients, Nilotinib (600mg/day) in 4 (4,87%) and Dasatinib (100mg/day) in 3 (3.65%). We had 5 patients with Imatinib (800/day), 1 with Bosutinib (500mg/day) and 1 with Bosutinib (400/day), all included in a clinical trial. Low-dose TKIs was started in 6 patients due to age (median age of 78.3, range 50-87); and comorbidities. 4 patients (4,94%) took Imatinib 300mg/day and 2 (2,47%), Imatinib 200mg/day. The main comorbidities for low initial dose were ischemic heart disease, atrial fibrillation, renal failure and vasculopathy. We lost follow up of 4 patients, finally analyzing the response to treatment only in 72 patients, standard dose versus patients who required adjustment the following years.
During this period, 33 (40,2%) patients, with a median age of 66.44 years, required TKIs dose reduction: 17 (51.6%) due to toxicity, and 16 of them (48.4%) in order to prevent long term toxicities once they achieved a MR ≥ 4.
Although 41 patients (50%) presented toxicities, only 17 required dose adjustment. We found mild-to-moderate events: diarrhea in 5 patients, arthromyalgia in 4, hematological toxicity in 3, cutaneous in 5, mainly skin rash, acute pulmonary edema in 1 patient and edema in another one. Ten patients from the initial population required to change first-line TKI treatment due to toxicities: 8 patients received a different TKI as a second-line treatment, whereas 2 of them changed to cytoreductive therapy.
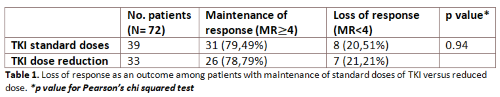
In table 1, we describe the proportion of patients who lost response (MR<4): 21.21% in the group of low dose and 20.51% in the group of standard dose. When comparing TKIs dose reduction group with the group which maintained standard dose, we found no statistically differences in terms of loss of response (MR<4), using the Fisher’s exact test (p 0.94).
Conclusion
Even if only 33 patients required a dose reduction, 26 (78.79%) maintained the depth of the molecular response (MR≥4). We can conclude that, if required, due to adverse events or in order to reduce long-term toxicities, the reduction of TKI has a clear clinical benefit and an impact on life quality, without the lost of a deep molecular response.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: PB1518
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
Long-term survival in chronic myeloid leukemia (CML) has been achieved with the advent of the BCR-ABL tyrosine kinase inhibitors (TKIs), which represent the standard treatment of this disease nowadays. However, almost 20 % of LMC patients present TKIs intolerance, and finding the right dose while maintaining an optimal response, without therapy-related adverse events, is challenging.
Aims
The aim of our study was to retrospectively analyze the impact of low dose TKIs on the deep molecular response (MR) in the group of patients who required a dose reduction due to adverse effects or in order to prevent long-term toxicities, in comparison with those who maintained standard dose.
Methods
We analyzed 82 patients diagnosed with chronic phase of CML from January 2000 to December 2020, who started treatment with TKIs.
Results
Among the population of study, 47 (58,02%) were male and 35 (42,6%) female, with a median age of 50 years (range 13-87ys), at the moment of diagnosis.
Standard dose of Imatinib (400mg/day) was the first line TKI in 63 (76,8%) patients, Nilotinib (600mg/day) in 4 (4,87%) and Dasatinib (100mg/day) in 3 (3.65%). We had 5 patients with Imatinib (800/day), 1 with Bosutinib (500mg/day) and 1 with Bosutinib (400/day), all included in a clinical trial. Low-dose TKIs was started in 6 patients due to age (median age of 78.3, range 50-87); and comorbidities. 4 patients (4,94%) took Imatinib 300mg/day and 2 (2,47%), Imatinib 200mg/day. The main comorbidities for low initial dose were ischemic heart disease, atrial fibrillation, renal failure and vasculopathy. We lost follow up of 4 patients, finally analyzing the response to treatment only in 72 patients, standard dose versus patients who required adjustment the following years.
During this period, 33 (40,2%) patients, with a median age of 66.44 years, required TKIs dose reduction: 17 (51.6%) due to toxicity, and 16 of them (48.4%) in order to prevent long term toxicities once they achieved a MR ≥ 4.
Although 41 patients (50%) presented toxicities, only 17 required dose adjustment. We found mild-to-moderate events: diarrhea in 5 patients, arthromyalgia in 4, hematological toxicity in 3, cutaneous in 5, mainly skin rash, acute pulmonary edema in 1 patient and edema in another one. Ten patients from the initial population required to change first-line TKI treatment due to toxicities: 8 patients received a different TKI as a second-line treatment, whereas 2 of them changed to cytoreductive therapy.
In table 1, we describe the proportion of patients who lost response (MR<4): 21.21% in the group of low dose and 20.51% in the group of standard dose. When comparing TKIs dose reduction group with the group which maintained standard dose, we found no statistically differences in terms of loss of response (MR<4), using the Fisher’s exact test (p 0.94).

Conclusion
Even if only 33 patients required a dose reduction, 26 (78.79%) maintained the depth of the molecular response (MR≥4). We can conclude that, if required, due to adverse events or in order to reduce long-term toxicities, the reduction of TKI has a clear clinical benefit and an impact on life quality, without the lost of a deep molecular response.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor


