Contributions
Abstract: PB1517
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
The availability of real-world data of patients with chronic myeloid leukemia (CML) collected outside the context of clinical trials is limited.
Aims
To describe the use and choice of tyrosine kinase inhibitors (TKIs), the achievement of response per European LeukemiaNet (ELN) criteria as well as adherence to ELN recommendations in this unselected patient population in Switzerland.
Methods
This non-interventional, multicenter, retrospective chart review analyzed data from patients with newly diagnosed CML treated at six Swiss hospitals. Written informed consent was obtained from all individual participants before data collection. Included patients had received front-line TKI and were solely treated with TKIs between 2010–2015, with a minimum follow-up of 18 months. Treatment efficacy was evaluated according to ELN 2013 milestone achievements at predefined time points (3, 6, 12 and 18 months, last follow-up).
Results
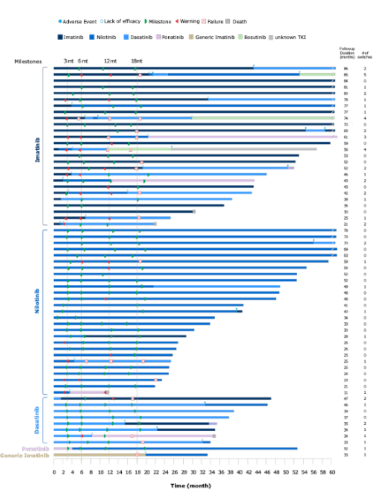
Data from 63 patients treated with TKIs were collected (first-line imatinib [n = 27], nilotinib [n = 27], dasatinib [n = 8], or ponatinib [n = 1]); 56% of patients were men, and the median age at diagnosis was 55 years. A high frequency of TKI therapy switches (49 times) and treatment changes (dose adjustment, dose interruption or re-initiation; 165 times) due to intolerance or insufficient response were recorded. First-line treatment with nilotinib or dasatinib compared with imatinib consistently resulted in a higher proportion of patients achieving an ELN-defined optimal response at every timepoint, irrespective of subsequent TKI therapy. A major molecular response (BCR-ABLIS ≤ 0.1%) was not reached at last visit for 18% of patients irrespective of TKI and for 24% of patients treated with imatinib as first-line therapy. A higher percentage of patients in the nilotinib and dasatinib groups versus the imatinib group reached a deep molecular response (BCR-ABL1IS ≤ 0.01%) at 12 months (32% and 25%, respectively, vs. 23%) and 18 months (42% and 38%, respectively, vs 27%). Patients who received nilotinib or dasatinib as front-line TKI switched therapies less frequently compared with patients treated with first-line imatinib, irrespective of subsequent TKI therapy.
Conclusion
This study confirms that in a real-world patient population ELN guidelines are generally implemented in Switzerland. While TKI use involves all approved inhibitors, with a large proportion of patients achieving ELN milestones, an unexpectedly high number of TKI therapy switches suggests a clear difference in TKI use between registration trials and clinical practice.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor
Abstract: PB1517
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
The availability of real-world data of patients with chronic myeloid leukemia (CML) collected outside the context of clinical trials is limited.
Aims
To describe the use and choice of tyrosine kinase inhibitors (TKIs), the achievement of response per European LeukemiaNet (ELN) criteria as well as adherence to ELN recommendations in this unselected patient population in Switzerland.
Methods
This non-interventional, multicenter, retrospective chart review analyzed data from patients with newly diagnosed CML treated at six Swiss hospitals. Written informed consent was obtained from all individual participants before data collection. Included patients had received front-line TKI and were solely treated with TKIs between 2010–2015, with a minimum follow-up of 18 months. Treatment efficacy was evaluated according to ELN 2013 milestone achievements at predefined time points (3, 6, 12 and 18 months, last follow-up).
Results
Data from 63 patients treated with TKIs were collected (first-line imatinib [n = 27], nilotinib [n = 27], dasatinib [n = 8], or ponatinib [n = 1]); 56% of patients were men, and the median age at diagnosis was 55 years. A high frequency of TKI therapy switches (49 times) and treatment changes (dose adjustment, dose interruption or re-initiation; 165 times) due to intolerance or insufficient response were recorded. First-line treatment with nilotinib or dasatinib compared with imatinib consistently resulted in a higher proportion of patients achieving an ELN-defined optimal response at every timepoint, irrespective of subsequent TKI therapy. A major molecular response (BCR-ABLIS ≤ 0.1%) was not reached at last visit for 18% of patients irrespective of TKI and for 24% of patients treated with imatinib as first-line therapy. A higher percentage of patients in the nilotinib and dasatinib groups versus the imatinib group reached a deep molecular response (BCR-ABL1IS ≤ 0.01%) at 12 months (32% and 25%, respectively, vs. 23%) and 18 months (42% and 38%, respectively, vs 27%). Patients who received nilotinib or dasatinib as front-line TKI switched therapies less frequently compared with patients treated with first-line imatinib, irrespective of subsequent TKI therapy.
Conclusion
This study confirms that in a real-world patient population ELN guidelines are generally implemented in Switzerland. While TKI use involves all approved inhibitors, with a large proportion of patients achieving ELN milestones, an unexpectedly high number of TKI therapy switches suggests a clear difference in TKI use between registration trials and clinical practice.
Keyword(s): Chronic myeloid leukemia, Tyrosine kinase inhibitor