
Contributions
Abstract: PB1516
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
The introduction of Imatinib in the treatment of Chronic Myeloid Leukemia (CML) changed radically the outcome of patients, transforming a fatal disease to a disease compatible with normal lifespan. Currently, 4 tyrosine kinase inhibitors (TKIs) have been approved for the treatment of newly diagnosed CML patients in chronic phase (CP). Treatment goal is the deepest molecular response. However, the optimal time for this achievement that results in the best long-term outcome and the best chance for treatment free remission remains under discussion. Patients that do not achieve early molecular response (EMR) define a group with unfavorable prognosis. The optimal choice of TKI in terms of both effectiveness and safety remains a challenge.
Aims
In this retrospective real-world study, our goal is to investigate the role of EMR in the long-term outcome in regard to the TKI that was administered as first line treatment.
Methods
261 patients with CML-CP that were diagnosed between 2005-2020 were included. Patients were treated with either 1st or 2nd generation TKI according to treating physician’s choice and availability at the time of diagnosis. Regular evaluation of molecular response with RQ-PCR was done according to ELN guidelines. EMR (≤10% IS at 3 months) and the cumulative incidence (CI) of achievement of MMR and MR4.0 in regard to selected TKI were estimated. Event free survival (EFS) was defined as the time from treatment onset to death, disease progression, treatment failure or treatment change.
Results
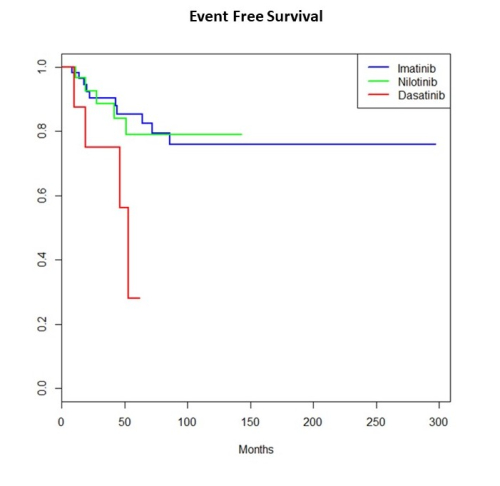
Data for EMR were available for 148 patients and were included in the analysis. First line TKI was imatinib (IM) in 94, nilotinib (NIL) in 44 and dasatinib (DAS) in 10 cases. Prior to completion of three consecutive months of treatment 6 patients changed TKI due to intolerance/toxicities (IM, n=4 due to pyrexia, myalgias, edema, toxic epidermal necrolysis – NIL, n=1 due to heart failure – DAS, n=1 due to pulmonary hypertension). EMR was evaluated to the remaining 142 patients. Overall, EMR achieved 76.6%, 80.9% and 100% of patients on IM, NIL and DAS respectively (p=NS). Of 113 patients that achieved EMR 107 were on the same TKI 12 months after diagnosis. All TKI changes prior to 12 months were made due to grade ≥3 toxicities (IM, n=5 due to rash, edema, myalgias and NIL, n=1 due to gout and rash). At this timepoint, the CI of MMR was 67.7%, 70.6% and 90% for IM, NIL and DAS, respectively (p=0.06). On the same TKI remained at least for 18 consecutive months 103 patients. Three patients were switched due to toxicities (rash on IM, myocardial infarction on NIL, pleural effusion on DAS) and one patient due to not optimal treatment response on NIL according to ELN2020 (BCR/ABL 0.52% IS). The CI of MR4.0 at this timepoint was 45.4%, 50.9% and 73.3% on IM, NIL and DAS, respectively (p=0.16). After a median follow-up of 70.6 months the median has not been reached. At 60 months the EFS was 85.3%, 78.9% and 28.1% for IM, NIL and DAS, respectively (p=0.02). Of note, 69.9% of patients in our initial cohort achieved EMR and remained on treatment with IM for ≥18 months.
Conclusion
Long-term EFS depends on both the effectiveness and the safety profile of the TKI. In terms of effectiveness, EMR can provide guidance at an early timepoint. Although more potent TKIs are available, IM still appears to be satisfactory as frontline treatment choice taking into consideration the long term safety profile.
Keyword(s): BCR-ABL, Tyrosine kinase inhibitor
Abstract: PB1516
Type: Publication Only
Session title: Chronic myeloid leukemia - Clinical
Background
The introduction of Imatinib in the treatment of Chronic Myeloid Leukemia (CML) changed radically the outcome of patients, transforming a fatal disease to a disease compatible with normal lifespan. Currently, 4 tyrosine kinase inhibitors (TKIs) have been approved for the treatment of newly diagnosed CML patients in chronic phase (CP). Treatment goal is the deepest molecular response. However, the optimal time for this achievement that results in the best long-term outcome and the best chance for treatment free remission remains under discussion. Patients that do not achieve early molecular response (EMR) define a group with unfavorable prognosis. The optimal choice of TKI in terms of both effectiveness and safety remains a challenge.
Aims
In this retrospective real-world study, our goal is to investigate the role of EMR in the long-term outcome in regard to the TKI that was administered as first line treatment.
Methods
261 patients with CML-CP that were diagnosed between 2005-2020 were included. Patients were treated with either 1st or 2nd generation TKI according to treating physician’s choice and availability at the time of diagnosis. Regular evaluation of molecular response with RQ-PCR was done according to ELN guidelines. EMR (≤10% IS at 3 months) and the cumulative incidence (CI) of achievement of MMR and MR4.0 in regard to selected TKI were estimated. Event free survival (EFS) was defined as the time from treatment onset to death, disease progression, treatment failure or treatment change.
Results
Data for EMR were available for 148 patients and were included in the analysis. First line TKI was imatinib (IM) in 94, nilotinib (NIL) in 44 and dasatinib (DAS) in 10 cases. Prior to completion of three consecutive months of treatment 6 patients changed TKI due to intolerance/toxicities (IM, n=4 due to pyrexia, myalgias, edema, toxic epidermal necrolysis – NIL, n=1 due to heart failure – DAS, n=1 due to pulmonary hypertension). EMR was evaluated to the remaining 142 patients. Overall, EMR achieved 76.6%, 80.9% and 100% of patients on IM, NIL and DAS respectively (p=NS). Of 113 patients that achieved EMR 107 were on the same TKI 12 months after diagnosis. All TKI changes prior to 12 months were made due to grade ≥3 toxicities (IM, n=5 due to rash, edema, myalgias and NIL, n=1 due to gout and rash). At this timepoint, the CI of MMR was 67.7%, 70.6% and 90% for IM, NIL and DAS, respectively (p=0.06). On the same TKI remained at least for 18 consecutive months 103 patients. Three patients were switched due to toxicities (rash on IM, myocardial infarction on NIL, pleural effusion on DAS) and one patient due to not optimal treatment response on NIL according to ELN2020 (BCR/ABL 0.52% IS). The CI of MR4.0 at this timepoint was 45.4%, 50.9% and 73.3% on IM, NIL and DAS, respectively (p=0.16). After a median follow-up of 70.6 months the median has not been reached. At 60 months the EFS was 85.3%, 78.9% and 28.1% for IM, NIL and DAS, respectively (p=0.02). Of note, 69.9% of patients in our initial cohort achieved EMR and remained on treatment with IM for ≥18 months.

Conclusion
Long-term EFS depends on both the effectiveness and the safety profile of the TKI. In terms of effectiveness, EMR can provide guidance at an early timepoint. Although more potent TKIs are available, IM still appears to be satisfactory as frontline treatment choice taking into consideration the long term safety profile.
Keyword(s): BCR-ABL, Tyrosine kinase inhibitor


