
Contributions
Abstract: PB1494
Type: Publication Only
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Ibrutinib is increasingly used for treatment of various B-cell malignancies. It’s generally well tolerated, but has cardiovascular side-effect including hypertension and arrhythmias, most frequently atrial fibrillation (AFib). Data on the dynamics of frequency and severity of arrhythmias in patients on chronic ibrutinib treatment are lacking.
Aims
We initiated this prospective study to determine the impact of chronic ibrutinib therapy on incidence of supraventricular (SPB) and ventricular premature beats (VPB).
Methods
This was a multicentre, prospective, observational cohort study. Consecutive patients with hematologic malignancies starting ibrutinib therapy and without structural heart disease and clinically significant arrhythmias were included in the study. Cardiologic work-up included echocardiography and 24h-Holter-ECG. Diagnostic assessment was carried out before and repeated at 3, 6 and 12 months after the introduction of ibrutinib therapy. Primary endpoint was the number of VPBs on 24h-Holter-ECG; secondary endpoints were: number of SPBs on 24h-Holter-ECG, systolic left ventricular function, occurrence of AFib and newly diagnosed or worsened hypertension.
Results
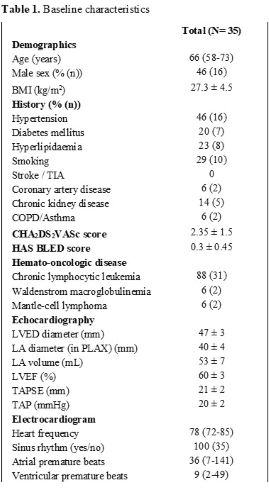
35 patients recruited from 4 hospitals were included in the study during 2019 and 2020 (Table 1). The most common indication for treatment was chronic lymphocytic leukaemia in 31 (89%) patients. Median age was 66 years (range 58-73), 54% were female. The most prevalent cardiovascular comorbidity was hypertension (45%), followed by smoking (29%). The remaining risk factors, including diabetes mellitus, stroke and coronary artery disease had a prevalence of <10%. Mean left ventricle ejection fraction was 60%, left atrial diameter 40±4 mm and volume 53±7 mL.After a median follow-up of 6 months, there was no significant increase in the number of VPBs (428±320 vs. 170±110, p=.19) or SPBs (245±104 vs. 532±305, p=.09). Hypertension was newly diagnosed or worsened in 8 patients (23%), and 2 patients developed paroxysmal AFib (6%). Ibrutinib was temporarily discontinued or its dose was adjusted in 3 patients due to non-cardiovascular side-effects.
Conclusion
Prolonged treatment with ibrutinib had no significant impact on number of VPBs or SPBs. Our study suggests that ibrutinib does not significantly increase the propensity to develop dangerous cardiac arrhythmias in patients who do not have clinically important structural heart disease or arrhythmias prior to treatment start and stresses the importance of hypertension control in this group of patients.
Keyword(s): Chronic lymphocytic leukemia, Ibrutinib, Side effects
Abstract: PB1494
Type: Publication Only
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Ibrutinib is increasingly used for treatment of various B-cell malignancies. It’s generally well tolerated, but has cardiovascular side-effect including hypertension and arrhythmias, most frequently atrial fibrillation (AFib). Data on the dynamics of frequency and severity of arrhythmias in patients on chronic ibrutinib treatment are lacking.
Aims
We initiated this prospective study to determine the impact of chronic ibrutinib therapy on incidence of supraventricular (SPB) and ventricular premature beats (VPB).
Methods
This was a multicentre, prospective, observational cohort study. Consecutive patients with hematologic malignancies starting ibrutinib therapy and without structural heart disease and clinically significant arrhythmias were included in the study. Cardiologic work-up included echocardiography and 24h-Holter-ECG. Diagnostic assessment was carried out before and repeated at 3, 6 and 12 months after the introduction of ibrutinib therapy. Primary endpoint was the number of VPBs on 24h-Holter-ECG; secondary endpoints were: number of SPBs on 24h-Holter-ECG, systolic left ventricular function, occurrence of AFib and newly diagnosed or worsened hypertension.
Results
35 patients recruited from 4 hospitals were included in the study during 2019 and 2020 (Table 1). The most common indication for treatment was chronic lymphocytic leukaemia in 31 (89%) patients. Median age was 66 years (range 58-73), 54% were female. The most prevalent cardiovascular comorbidity was hypertension (45%), followed by smoking (29%). The remaining risk factors, including diabetes mellitus, stroke and coronary artery disease had a prevalence of <10%. Mean left ventricle ejection fraction was 60%, left atrial diameter 40±4 mm and volume 53±7 mL.After a median follow-up of 6 months, there was no significant increase in the number of VPBs (428±320 vs. 170±110, p=.19) or SPBs (245±104 vs. 532±305, p=.09). Hypertension was newly diagnosed or worsened in 8 patients (23%), and 2 patients developed paroxysmal AFib (6%). Ibrutinib was temporarily discontinued or its dose was adjusted in 3 patients due to non-cardiovascular side-effects.

Conclusion
Prolonged treatment with ibrutinib had no significant impact on number of VPBs or SPBs. Our study suggests that ibrutinib does not significantly increase the propensity to develop dangerous cardiac arrhythmias in patients who do not have clinically important structural heart disease or arrhythmias prior to treatment start and stresses the importance of hypertension control in this group of patients.
Keyword(s): Chronic lymphocytic leukemia, Ibrutinib, Side effects


