Contributions
Abstract: PB1493
Type: Publication Only
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Pivotal clinical trials have shown Venetoclax (Ven; BCL inhibitor) good efficacy and safety profile. Studies in real life setting are limited as Ven is commercially available in Spain since April 2018.
Aims
To evaluate the effectiveness of Ven in adult CLL pts by the overall response rate (ORR) at 9 months (mo) after the first Ven dose administration, as assessed by the treating physician.
Methods
This is a Spanish non-interventional, retrospective, national, multicenter, post-marketing, observational study. Eligible subjects were adult pts with CLL who initiated Ven at least 9 mo before inclusion. Subjects in Early Access Program from Jan 2017 were included.
Data from approximately 100 pts is planned to be collected from 30 Spanish sites. Data of pts are retrospectively reviewed until the date of last follow-up or death.
Results
48 pts were included in the interim study: 32 pts (66.7%) were male, the median age was 74.5 years (67.5-78), pts had received a median of 4 prior lines of therapy (range 1-10 lines). 15 pts had unmutated IGHV (88%), 10 pts (33.3%) had del(17p), 10 pts (33.3%) had TP53 mut. In 42 pts, median time from CLL diagnosis to Ven initiation was 78.5 m (38-125).
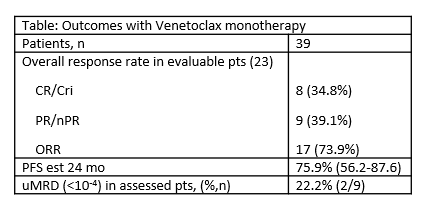
81.2% of pts received Ven monotherapy, 4.2% combined with rituximab, 2% with obinutuzumab,12.5% with other agents. Objective response was evaluated in 23/39 pts treated with Ven monotherapy.
48 pts (100.0%)had dose modifications, 19 (39.6%), dose interruptions, 19 (39.6%) discontinued Ven . 26 (54.2%) had TLS greater risk before Ven initiation, 22 pts were hospitalized during ramp-up.
14pts (29.2%) presented at least 1 SAE, 10 (20.8%) presented at least 1 SAE related to Ven. 6 pts (12.5%) presented 1 AE related to Ven that led to drug withdrawal. Percentages of Specific AEs were neutropenia (43.7%), febrile neutropenia (12.5%), serious infection (18.8%), TLS in 6.3% (2 laboratory,1 clinical) and Richter transformation (RT) (6.3%). Related to Ven were 4 febrile neutropenia (8.3%) and 1 lab TLS(2.1%).
Conclusion
This preliminary effectiveness data suggest overall outcomes are similar to those in the pivotal clinical trials. Safety assessment show AEs reported were consistent with the safety profile seen in prior VEN studies and did not reveal unexpected AEs.
Keyword(s): BCL2, Chronic lymphocytic leukemia, Clinical outcome, Relapse
Abstract: PB1493
Type: Publication Only
Session title: Chronic lymphocytic leukemia and related disorders - Clinical
Background
Pivotal clinical trials have shown Venetoclax (Ven; BCL inhibitor) good efficacy and safety profile. Studies in real life setting are limited as Ven is commercially available in Spain since April 2018.
Aims
To evaluate the effectiveness of Ven in adult CLL pts by the overall response rate (ORR) at 9 months (mo) after the first Ven dose administration, as assessed by the treating physician.
Methods
This is a Spanish non-interventional, retrospective, national, multicenter, post-marketing, observational study. Eligible subjects were adult pts with CLL who initiated Ven at least 9 mo before inclusion. Subjects in Early Access Program from Jan 2017 were included.
Data from approximately 100 pts is planned to be collected from 30 Spanish sites. Data of pts are retrospectively reviewed until the date of last follow-up or death.
Results
48 pts were included in the interim study: 32 pts (66.7%) were male, the median age was 74.5 years (67.5-78), pts had received a median of 4 prior lines of therapy (range 1-10 lines). 15 pts had unmutated IGHV (88%), 10 pts (33.3%) had del(17p), 10 pts (33.3%) had TP53 mut. In 42 pts, median time from CLL diagnosis to Ven initiation was 78.5 m (38-125).
81.2% of pts received Ven monotherapy, 4.2% combined with rituximab, 2% with obinutuzumab,12.5% with other agents. Objective response was evaluated in 23/39 pts treated with Ven monotherapy.
48 pts (100.0%)had dose modifications, 19 (39.6%), dose interruptions, 19 (39.6%) discontinued Ven . 26 (54.2%) had TLS greater risk before Ven initiation, 22 pts were hospitalized during ramp-up.
14pts (29.2%) presented at least 1 SAE, 10 (20.8%) presented at least 1 SAE related to Ven. 6 pts (12.5%) presented 1 AE related to Ven that led to drug withdrawal. Percentages of Specific AEs were neutropenia (43.7%), febrile neutropenia (12.5%), serious infection (18.8%), TLS in 6.3% (2 laboratory,1 clinical) and Richter transformation (RT) (6.3%). Related to Ven were 4 febrile neutropenia (8.3%) and 1 lab TLS(2.1%).
Conclusion
This preliminary effectiveness data suggest overall outcomes are similar to those in the pivotal clinical trials. Safety assessment show AEs reported were consistent with the safety profile seen in prior VEN studies and did not reveal unexpected AEs.
Keyword(s): BCL2, Chronic lymphocytic leukemia, Clinical outcome, Relapse