
Contributions
Abstract: PB1485
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to the C5 inhibitor eculizumab (ECU) after 16 weeks, improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al., EHA 2020).
Aims
We assessed the comparative effectiveness of PEG to the C5 inhibitor ravulizumab (RAV) using matching-adjusted indirect comparison (MAIC) methodology to conduct cross-trial comparisons.
Methods
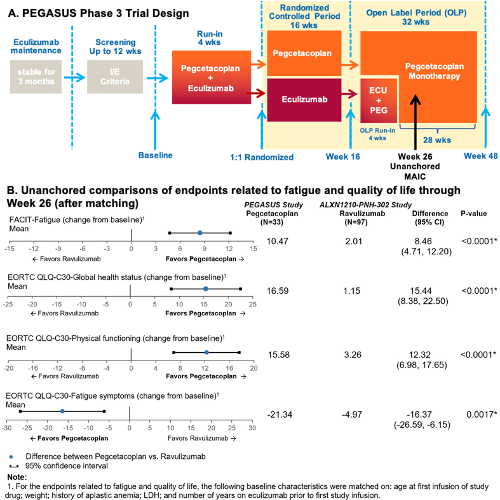
Individual patient data from PEGASUS were used to adjust for baseline differences compared to aggregate, published results from the randomized ALXN1210-PNH-302 (“302”) study (NCT03056040) which compared RAV and ECU at 26 weeks among patients with PNH with previous ECU treatment (Kulasekararaj et al., Blood 2019). In PEGASUS, patients completed a 16-week randomized controlled period (RCP) with 1:1 PEG and ECU randomization followed by PEG monotherapy for all patients for 28 weeks (Figure A). PEGASUS and 302 had similar eligibility criteria; however, PEGASUS required Hb <10.5 g/dL and absolute reticulocyte count >1x upper limit of normal while 302 did not. Propensity score weighting was used to balance demographic and clinical characteristics between the PEG arm from the PEGASUS trial and the RAV arm from the 302 trial. To compare to RAV results at Week 26, unanchored MAIC was used for PEGASUS patients from the RCP PEG group, using a proxy for Week 26 (mean of Week 24 and Week 28). Mean change from baseline (CFB) was calculated for Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score and for European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Scale (EORTC QLQ-C30) global health status, physical functioning, and fatigue symptoms questionnaires. Weighted Wald tests and 95% confidence intervals (CIs) were computed for comparisons of categorical and continuous outcomes.
Results
Thirty-three PEG patients from PEGASUS and 97 RAV patients from the 302 study were included. At Week 26, PEG patients experienced a clinically meaningful (>3 points) improvement in FACIT-Fatigue (higher score indicates less fatigue; mean CFB: 10.47 [95% CI: 6.98-13.95]) that was significantly greater (P<0.001) than the improvement for RAV patients (2.01 [95% CI: 0.64-3.38]) (Figure B). PEG patients also experienced clinically meaningful (>10 points) improvement in EORTC QLQ-30 global health status (higher score indicates improved patient health; mean CFB: 16.59 [95% CI: 10.34-22.84]) that was greater than in RAV patients (mean CFB: 1.15 [95% CI: -2.14-4.44], P<0.001). Similarly, PEG patients showed clinically meaningful (>10 pts) improvement in EORTC QLQ-30 physical functioning (mean CFB: 15.58 [95% CI: 10.53-20.62]), but RAV patients did not (mean CFB: 3.26 [95% CI: 1.53-4.99], P<0.001), indicating that PEG patients experienced higher levels of physical functioning. For EORTC QLQ-30 fatigue symptoms, in which a negative score indicates a decrease in fatigue, PEG patients experienced a clinically meaningful (>10 points) decrease in levels of fatigue (mean CFB: -21.34 [95% CI: -30.96 to -11.72]), but RAV patients did not (mean CFB: -4.97 [95% CI: -8.40 to -1.54], P<0.010).
Conclusion
MAIC results indicated that when compared with RAV, PEG treatment is associated with improvements in FACIT-Fatigue and EORTC QoL scores, evidencing an improvement in fatigue, physical functioning, global health, and quality of life.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Quality of life, Treatment
Abstract: PB1485
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to the C5 inhibitor eculizumab (ECU) after 16 weeks, improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al., EHA 2020).
Aims
We assessed the comparative effectiveness of PEG to the C5 inhibitor ravulizumab (RAV) using matching-adjusted indirect comparison (MAIC) methodology to conduct cross-trial comparisons.
Methods
Individual patient data from PEGASUS were used to adjust for baseline differences compared to aggregate, published results from the randomized ALXN1210-PNH-302 (“302”) study (NCT03056040) which compared RAV and ECU at 26 weeks among patients with PNH with previous ECU treatment (Kulasekararaj et al., Blood 2019). In PEGASUS, patients completed a 16-week randomized controlled period (RCP) with 1:1 PEG and ECU randomization followed by PEG monotherapy for all patients for 28 weeks (Figure A). PEGASUS and 302 had similar eligibility criteria; however, PEGASUS required Hb <10.5 g/dL and absolute reticulocyte count >1x upper limit of normal while 302 did not. Propensity score weighting was used to balance demographic and clinical characteristics between the PEG arm from the PEGASUS trial and the RAV arm from the 302 trial. To compare to RAV results at Week 26, unanchored MAIC was used for PEGASUS patients from the RCP PEG group, using a proxy for Week 26 (mean of Week 24 and Week 28). Mean change from baseline (CFB) was calculated for Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score and for European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Scale (EORTC QLQ-C30) global health status, physical functioning, and fatigue symptoms questionnaires. Weighted Wald tests and 95% confidence intervals (CIs) were computed for comparisons of categorical and continuous outcomes.
Results
Thirty-three PEG patients from PEGASUS and 97 RAV patients from the 302 study were included. At Week 26, PEG patients experienced a clinically meaningful (>3 points) improvement in FACIT-Fatigue (higher score indicates less fatigue; mean CFB: 10.47 [95% CI: 6.98-13.95]) that was significantly greater (P<0.001) than the improvement for RAV patients (2.01 [95% CI: 0.64-3.38]) (Figure B). PEG patients also experienced clinically meaningful (>10 points) improvement in EORTC QLQ-30 global health status (higher score indicates improved patient health; mean CFB: 16.59 [95% CI: 10.34-22.84]) that was greater than in RAV patients (mean CFB: 1.15 [95% CI: -2.14-4.44], P<0.001). Similarly, PEG patients showed clinically meaningful (>10 pts) improvement in EORTC QLQ-30 physical functioning (mean CFB: 15.58 [95% CI: 10.53-20.62]), but RAV patients did not (mean CFB: 3.26 [95% CI: 1.53-4.99], P<0.001), indicating that PEG patients experienced higher levels of physical functioning. For EORTC QLQ-30 fatigue symptoms, in which a negative score indicates a decrease in fatigue, PEG patients experienced a clinically meaningful (>10 points) decrease in levels of fatigue (mean CFB: -21.34 [95% CI: -30.96 to -11.72]), but RAV patients did not (mean CFB: -4.97 [95% CI: -8.40 to -1.54], P<0.010).

Conclusion
MAIC results indicated that when compared with RAV, PEG treatment is associated with improvements in FACIT-Fatigue and EORTC QoL scores, evidencing an improvement in fatigue, physical functioning, global health, and quality of life.
Keyword(s): Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Quality of life, Treatment


