
Contributions
Abstract: PB1480
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Crovalimab is a novel anti–complement C5 antibody currently being studied as a treatment for paroxysmal nocturnal hemoglobinuria (PNH), a life-threatening disease associated with hemolytic anemia and thrombosis. Treatment with approved C5 inhibitors eculizumab or ravulizumab is effective but can be limited by breakthrough hemolysis (BTH) due to unsustained C5 inhibition, inadequate efficacy in patients with C5 mutational variants and the requirement of regular intravenous (IV) infusions. Crovalimab is unique in that its properties allow for subcutaneous (SC) injections once every 4 weeks (Q4W) that can be self-administered. Additionally, crovalimab binds to C5 mutational variants. Promising results were obtained in the Phase I/II COMPOSER trial (NCT03157635; Röth et al, Blood. 2020) conducted in patients with PNH, with or without prior anti–C5 treatment.
Aims
The efficacy and safety of crovalimab vs eculizumab will be evaluated in two Phase III, randomized, open-label trials in patients with PNH, with or without current complement inhibition.
Methods
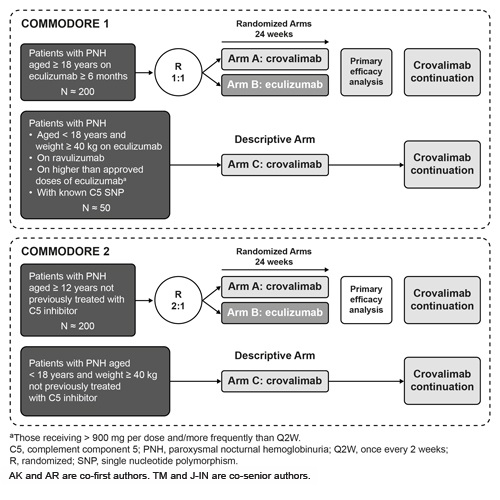
COMMODORE 1 (NCT04432584) will enroll patients who are receiving complement therapy. This trial is divided into 2 parts: 2 randomized arms (Arms A and B) for primary and secondary efficacy analyses and an exploratory descriptive arm (Arm C) (Figure). Patients eligible for the randomized arms will be randomized 1:1 to receive either crovalimab (Arm A) or eculizumab (Arm B). Arm A and C patients will receive crovalimab loading and subsequent SC Q4W maintenance dosing from week 5. Arm B patients will receive eculizumab IV maintenance dosing from D1 Q2W for a total of 24 weeks. Patients from each treatment arm can continue or switch to crovalimab after 24 weeks of treatment as determined by the treating physician. The primary efficacy objective is to determine the noninferiority of crovalimab vs eculizumab based on mean percent change in lactate dehydrogenase levels from baseline to week 25. Secondary efficacy objectives are to determine the proportion of patients who experience BTH, achieve transfusion avoidance (TA) or hemoglobin stabilization, as well as determine mean change in fatigue according to the Functional Assessment of Chronic Illness Therapy–Fatigue questionnaire from baseline to week 25. Safety and tolerability of crovalimab will also be evaluated along with pharmacokinetic, immunogenicity and health status utility objectives.
COMMODORE 2 (NCT04434092) will enroll patients not previously treated with complement inhibitors. This trial is also divided into 2 parts (Figure). Patients aged ≥ 12 years will be randomized 2:1 to receive either crovalimab (Arm A) or eculizumab (Arm B). Arm A and C patients will receive crovalimab loading and subsequent SC Q4W maintenance dosing from week 5. Arm B patients will receive induction doses of eculizumab IV QW for 4 weeks followed by maintenance dosing Q2W up to 24 weeks. Patients from each treatment arm can continue or switch to crovalimab as determined by the treating physician. The co-primary efficacy objectives are to determine the noninferiority of crovalimab vs eculizumab, based on the proportion of patients 1) who achieve TA from baseline to week 25 and 2) with hemolysis control from week 5-25. Safety and tolerability of crovalimab will also be evaluated along with pharmacokinetic, immunogenicity and health status utility objectives.
Results
COMMODORE 1 and 2 are now enrolling.
Conclusion
The COMMODORE 1 and 2 studies aim to assess the efficacy and safety of crovalimab in patients with PNH, compared with eculizumab, in patients with or without prior complement inhibition.
Keyword(s): Complement, Hemolysis, Monoclonal antibody, Paroxysmal nocturnal hemoglobinuria (PNH)
Abstract: PB1480
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Crovalimab is a novel anti–complement C5 antibody currently being studied as a treatment for paroxysmal nocturnal hemoglobinuria (PNH), a life-threatening disease associated with hemolytic anemia and thrombosis. Treatment with approved C5 inhibitors eculizumab or ravulizumab is effective but can be limited by breakthrough hemolysis (BTH) due to unsustained C5 inhibition, inadequate efficacy in patients with C5 mutational variants and the requirement of regular intravenous (IV) infusions. Crovalimab is unique in that its properties allow for subcutaneous (SC) injections once every 4 weeks (Q4W) that can be self-administered. Additionally, crovalimab binds to C5 mutational variants. Promising results were obtained in the Phase I/II COMPOSER trial (NCT03157635; Röth et al, Blood. 2020) conducted in patients with PNH, with or without prior anti–C5 treatment.
Aims
The efficacy and safety of crovalimab vs eculizumab will be evaluated in two Phase III, randomized, open-label trials in patients with PNH, with or without current complement inhibition.
Methods
COMMODORE 1 (NCT04432584) will enroll patients who are receiving complement therapy. This trial is divided into 2 parts: 2 randomized arms (Arms A and B) for primary and secondary efficacy analyses and an exploratory descriptive arm (Arm C) (Figure). Patients eligible for the randomized arms will be randomized 1:1 to receive either crovalimab (Arm A) or eculizumab (Arm B). Arm A and C patients will receive crovalimab loading and subsequent SC Q4W maintenance dosing from week 5. Arm B patients will receive eculizumab IV maintenance dosing from D1 Q2W for a total of 24 weeks. Patients from each treatment arm can continue or switch to crovalimab after 24 weeks of treatment as determined by the treating physician. The primary efficacy objective is to determine the noninferiority of crovalimab vs eculizumab based on mean percent change in lactate dehydrogenase levels from baseline to week 25. Secondary efficacy objectives are to determine the proportion of patients who experience BTH, achieve transfusion avoidance (TA) or hemoglobin stabilization, as well as determine mean change in fatigue according to the Functional Assessment of Chronic Illness Therapy–Fatigue questionnaire from baseline to week 25. Safety and tolerability of crovalimab will also be evaluated along with pharmacokinetic, immunogenicity and health status utility objectives.
COMMODORE 2 (NCT04434092) will enroll patients not previously treated with complement inhibitors. This trial is also divided into 2 parts (Figure). Patients aged ≥ 12 years will be randomized 2:1 to receive either crovalimab (Arm A) or eculizumab (Arm B). Arm A and C patients will receive crovalimab loading and subsequent SC Q4W maintenance dosing from week 5. Arm B patients will receive induction doses of eculizumab IV QW for 4 weeks followed by maintenance dosing Q2W up to 24 weeks. Patients from each treatment arm can continue or switch to crovalimab as determined by the treating physician. The co-primary efficacy objectives are to determine the noninferiority of crovalimab vs eculizumab, based on the proportion of patients 1) who achieve TA from baseline to week 25 and 2) with hemolysis control from week 5-25. Safety and tolerability of crovalimab will also be evaluated along with pharmacokinetic, immunogenicity and health status utility objectives.
Results
COMMODORE 1 and 2 are now enrolling.

Conclusion
The COMMODORE 1 and 2 studies aim to assess the efficacy and safety of crovalimab in patients with PNH, compared with eculizumab, in patients with or without prior complement inhibition.
Keyword(s): Complement, Hemolysis, Monoclonal antibody, Paroxysmal nocturnal hemoglobinuria (PNH)


