
Contributions
Abstract: PB1478
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The bone marrow failure diseases include a group of disorders that can be either inherited or acquired, involving either 1 cell line or all of the cell lines. Acquired aplastic anemia and myelodysplastic syndrome are common diseases, manifested as bone marrow failure. Acquired aplastic anemia (aAA) is thought to be driven by Human Leukocyte Antigen (HLA)-restricted T cell immunity, with earlier studies favoring HLA class II-mediated pathways. Owing to the association of autoimmune disease with pathognomonic HLA, we hypothesized that part of the MDS and aAA patients could associate with HLA genotype and thus those patients are prone to develop bone marrow failure diseases.
Aims
To characterize the association between HLA genotype and bone marrow failure disease, we therefore designed a study to investigate the association of HLA genotype and its correlation with bone marrow failure disease.
Methods
A total of 12 patients with acquired severe aplastic anemia (aSAA) and 10 patients with myelodysplastic anemia (MDS) were enrolled in this study, as well as 10 health controls. The enrolled patients were diagnosed in our department from 2019.07 to 2020.12. All subjects underwent blood sampling on EDTA as anti-coagulant for DNA extraction. We used a next-generation sequencer (MiSeq, Illumina, CA, USA) to identify the HLA of HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DP, and HLA-DQ genes in each patient and heath control. Informed consent was obtained from the study participants for the genetic analyses. The diagnosis and severity of AA and MDS were determined according to standard criteria. Tests of proportions were used to compare allelic frequencies.
Results
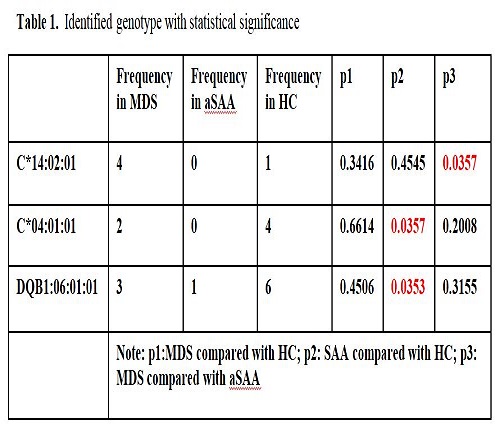
We found HLA-C*14:02:01, HLA-C*04:01:01, HLA-DQB1*06:01:01 might be involved in the pathogenesis in bone marrow failure diseases (all p<0.05). As for HLA-C*14:02:01, frequency of this gene in MDS and SAA is 20% and 0%, respectively. (p=0.0357) This serotype of HLA-C might be a biomarker to distinguish MDS from SAA. As for HLA-C*04:01:01, frequency of this gene in SAA and HC is 0% and 20%, respectively. (p=0.0357) This serotype of HLA-C might be a major protective factor in the pathogenesis of aSAA. Similarly, the frequency of HLA-DQB1*06:01:01 in SAA and HC is 4.17% and 30%, respectively. (p=0.0353) This serotype of HLA-DQ might be a major protective factor in the pathogenesis of aSAA (Table 1). Supporting by previous study, we found high risk HLA-DR alleles such as DRB1*15:01 is highly expressed in patients with MDS or SAA (35%, 41.6%, respectively), compared with health control (25%). However, we did not find a statistical significance between three groups.
Conclusion
HLA genotype could be a surrogate marker to monitor the disease severity and predisposition to bone marrow failure disease.
Keyword(s): Aplastic anemia, Bone marrow failure, Myelodysplasia
Abstract: PB1478
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The bone marrow failure diseases include a group of disorders that can be either inherited or acquired, involving either 1 cell line or all of the cell lines. Acquired aplastic anemia and myelodysplastic syndrome are common diseases, manifested as bone marrow failure. Acquired aplastic anemia (aAA) is thought to be driven by Human Leukocyte Antigen (HLA)-restricted T cell immunity, with earlier studies favoring HLA class II-mediated pathways. Owing to the association of autoimmune disease with pathognomonic HLA, we hypothesized that part of the MDS and aAA patients could associate with HLA genotype and thus those patients are prone to develop bone marrow failure diseases.
Aims
To characterize the association between HLA genotype and bone marrow failure disease, we therefore designed a study to investigate the association of HLA genotype and its correlation with bone marrow failure disease.
Methods
A total of 12 patients with acquired severe aplastic anemia (aSAA) and 10 patients with myelodysplastic anemia (MDS) were enrolled in this study, as well as 10 health controls. The enrolled patients were diagnosed in our department from 2019.07 to 2020.12. All subjects underwent blood sampling on EDTA as anti-coagulant for DNA extraction. We used a next-generation sequencer (MiSeq, Illumina, CA, USA) to identify the HLA of HLA-A, HLA-B, HLA-C, HLA-DR, HLA-DP, and HLA-DQ genes in each patient and heath control. Informed consent was obtained from the study participants for the genetic analyses. The diagnosis and severity of AA and MDS were determined according to standard criteria. Tests of proportions were used to compare allelic frequencies.
Results
We found HLA-C*14:02:01, HLA-C*04:01:01, HLA-DQB1*06:01:01 might be involved in the pathogenesis in bone marrow failure diseases (all p<0.05). As for HLA-C*14:02:01, frequency of this gene in MDS and SAA is 20% and 0%, respectively. (p=0.0357) This serotype of HLA-C might be a biomarker to distinguish MDS from SAA. As for HLA-C*04:01:01, frequency of this gene in SAA and HC is 0% and 20%, respectively. (p=0.0357) This serotype of HLA-C might be a major protective factor in the pathogenesis of aSAA. Similarly, the frequency of HLA-DQB1*06:01:01 in SAA and HC is 4.17% and 30%, respectively. (p=0.0353) This serotype of HLA-DQ might be a major protective factor in the pathogenesis of aSAA (Table 1). Supporting by previous study, we found high risk HLA-DR alleles such as DRB1*15:01 is highly expressed in patients with MDS or SAA (35%, 41.6%, respectively), compared with health control (25%). However, we did not find a statistical significance between three groups.

Conclusion
HLA genotype could be a surrogate marker to monitor the disease severity and predisposition to bone marrow failure disease.
Keyword(s): Aplastic anemia, Bone marrow failure, Myelodysplasia


