
Contributions
Abstract: PB1477
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with paroxysmal nocturnal hemoglobinuria (PNH) (Hillmen P et al, EHA 2020).
Aims
This post-hoc analysis categorized the hematologic response to PEG versus ECU treatment in PEGASUS patients with PNH up to Week 48.
Methods
Hematologic response to PEG or ECU in PEGASUS patients (≥18 years) with PNH and Hb <10.5 g/dL (despite stable ECU treatment for ≥3 months) was categorized as complete, major, good, partial, minor, or no response at baseline, Week 16, and Week 48 using the number of packed red blood cell transfusions required, Hb level, lactate dehydrogenase (LDH) level, and absolute reticulocyte count assessed in the last 6 months of treatment (ARC; categorization criteria per Risitano AM, et al. Front Immunol. 2019;10:1157). Hematologic response criteria are as follows: Complete response, no transfusions required, stable Hb (normal range), and no evidence of hemolysis (LDH≤1.5×upper limit of normal [ULN] U/L, ARC≤150,000/µL); Major response: no transfusion, normal Hb, but with evidence of hemolysis (LDH>1.5×ULN U/L and/or ARC>150,000/µL); Good response: no transfusions, but with chronic mild anemia or evidence of hemolysis; Partial response: chronic moderate anemia and/or occasional transfusions (<3 units/6 months); Minor response: regular transfusions required (3-6 units/6 months); No response: regular and frequent transfusions required (>6 units/6 months). Statistical tests were performed to determine significance for patient responses of ‘Good,’ ‘Major,’ or ‘Complete’, collectively (McNemar’s test within arms; Chi-squared between arms).
Results
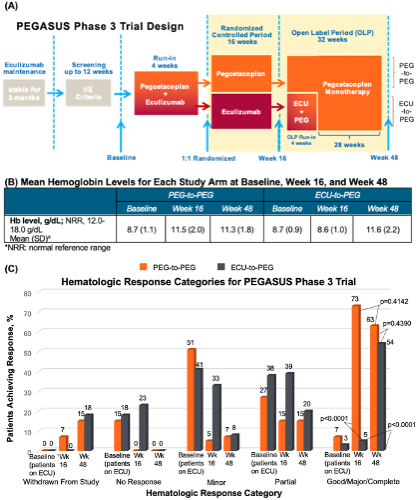
Of 80 enrolled patients, 41 and 39 were randomized to the PEG-to-PEG and ECU-to-PEG study arms, respectively (Figure A). Mean Hb levels for each arm at baseline, Week 16, and Week 48 are shown below (Figure B). At baseline, 7% (PEG-to-PEG) and 3% (ECU-to-PEG) of patients exhibited a Good/Major/Complete hematologic response. At Week 16, 73% (PEG-to-PEG) and 5% (ECU-to-PEG) of patients exhibited this response (p<0.0001), while the proportion of patients who achieved this response was similar between arms at Week 48 (p=0.4390). In total at Week 48, 63% of patients in the PEG-to-PEG arm (p=0.4142 vs. Week 16) and 54% of patients in the ECU-to-PEG arm (p<0.0001 vs. Week 16) achieved a Good/Major/Complete hematologic response, demonstrating a significant increase in the percent of ECU-to-PEG patients achieving at least a Good response after switching to PEG (Figure C).
Overall, 13 left the study; 12 patients discontinued PEG: 3 in the randomized controlled period, 8 in the open label period due to TEAEs (6 in ECU-to-PEG, 2 in PEG-to-PEG), and one during follow-up; one additional patient withdrew due to physician decision. These 13 patients (12 discontinuations and 1 withdrawal) were not included in statistical analyses.
Conclusion
In this post-hoc analysis of the PEGASUS study, PEG treatment resulted in a significantly greater proportion of patients with Good or better hematologic responses after switching from ECU treatment. These results further support that proximal complement inhibition, by preventing EVH in addition to controlling IVH, leads to clinical and hematologic improvement in PNH treatment.
Keyword(s): Hemoglobin, Paroxysmal nocturnal hemoglobinuria (PNH), Phase III, Progression
Abstract: PB1477
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with paroxysmal nocturnal hemoglobinuria (PNH) (Hillmen P et al, EHA 2020).
Aims
This post-hoc analysis categorized the hematologic response to PEG versus ECU treatment in PEGASUS patients with PNH up to Week 48.
Methods
Hematologic response to PEG or ECU in PEGASUS patients (≥18 years) with PNH and Hb <10.5 g/dL (despite stable ECU treatment for ≥3 months) was categorized as complete, major, good, partial, minor, or no response at baseline, Week 16, and Week 48 using the number of packed red blood cell transfusions required, Hb level, lactate dehydrogenase (LDH) level, and absolute reticulocyte count assessed in the last 6 months of treatment (ARC; categorization criteria per Risitano AM, et al. Front Immunol. 2019;10:1157). Hematologic response criteria are as follows: Complete response, no transfusions required, stable Hb (normal range), and no evidence of hemolysis (LDH≤1.5×upper limit of normal [ULN] U/L, ARC≤150,000/µL); Major response: no transfusion, normal Hb, but with evidence of hemolysis (LDH>1.5×ULN U/L and/or ARC>150,000/µL); Good response: no transfusions, but with chronic mild anemia or evidence of hemolysis; Partial response: chronic moderate anemia and/or occasional transfusions (<3 units/6 months); Minor response: regular transfusions required (3-6 units/6 months); No response: regular and frequent transfusions required (>6 units/6 months). Statistical tests were performed to determine significance for patient responses of ‘Good,’ ‘Major,’ or ‘Complete’, collectively (McNemar’s test within arms; Chi-squared between arms).
Results
Of 80 enrolled patients, 41 and 39 were randomized to the PEG-to-PEG and ECU-to-PEG study arms, respectively (Figure A). Mean Hb levels for each arm at baseline, Week 16, and Week 48 are shown below (Figure B). At baseline, 7% (PEG-to-PEG) and 3% (ECU-to-PEG) of patients exhibited a Good/Major/Complete hematologic response. At Week 16, 73% (PEG-to-PEG) and 5% (ECU-to-PEG) of patients exhibited this response (p<0.0001), while the proportion of patients who achieved this response was similar between arms at Week 48 (p=0.4390). In total at Week 48, 63% of patients in the PEG-to-PEG arm (p=0.4142 vs. Week 16) and 54% of patients in the ECU-to-PEG arm (p<0.0001 vs. Week 16) achieved a Good/Major/Complete hematologic response, demonstrating a significant increase in the percent of ECU-to-PEG patients achieving at least a Good response after switching to PEG (Figure C).
Overall, 13 left the study; 12 patients discontinued PEG: 3 in the randomized controlled period, 8 in the open label period due to TEAEs (6 in ECU-to-PEG, 2 in PEG-to-PEG), and one during follow-up; one additional patient withdrew due to physician decision. These 13 patients (12 discontinuations and 1 withdrawal) were not included in statistical analyses.

Conclusion
In this post-hoc analysis of the PEGASUS study, PEG treatment resulted in a significantly greater proportion of patients with Good or better hematologic responses after switching from ECU treatment. These results further support that proximal complement inhibition, by preventing EVH in addition to controlling IVH, leads to clinical and hematologic improvement in PNH treatment.
Keyword(s): Hemoglobin, Paroxysmal nocturnal hemoglobinuria (PNH), Phase III, Progression


