
Contributions
Abstract: PB1476
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The standard of care in PNH is anti-C5 monoclonal antibody (MAb) treatment (tx) with eculizumab (ECU) or ravulizumab (RAV) but hematological responses can be heterogenous. Iptacopan (LNP023) is a first-in-class, oral, small-molecule, selective and reversible complement factor B inhibitor that inhibits both intra- and extravascular hemolysis. In an ongoing Ph2 study (NCT03439839), iptacopan add-on tx has demonstrated improvements in hematological response and biomarkers of disease activity in adult patients (pts) with PNH with active hemolysis despite ECU tx.
Aims
The Ph3 APPLY-PNH study (NCT04558918) will assess the efficacy and safety of iptacopan monotherapy in adult pts with PNH with residual anemia despite prior anti-C5 MAb tx by evaluating its superiority to anti-C5 MAb tx.
Methods
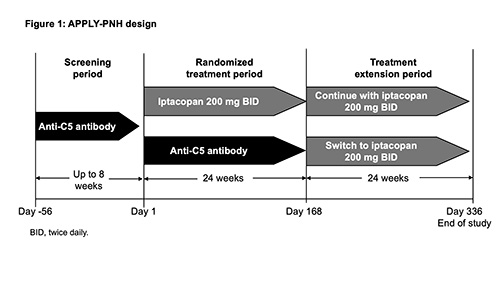
The study comprises an ≤8-wk screening period; a 24-wk, randomized, open‑label, active‑controlled, parallel‑group tx period; and a 24-wk tx extension period (Fig. 1). Pts (N≈91) will be randomized (8:5) to iptacopan monotherapy 200mg orally BID (seamless switch from prior tx) or intravenous ECU/RAV (continue prior tx). Pts will be stratified based on prior anti-C5 tx and transfusion history. On completion of the Wk 24 visit, pts may enter the 24-wk iptacopan tx extension period. Eligible pts must have a confirmed diagnosis of PNH (red blood cells [RBCs] and white blood cells [granulocyte/monocyte] clone size ≥10%) and residual anemia (mean Hb <10g/dL) despite receiving a stable regimen of ECU/RAV in the 6 months preceding randomization. Key exclusion criteria include ECU dosing interval ≤11 days; known or suspected hereditary complement deficiency; hematopoietic stem cell transplantation; laboratory evidence of bone marrow failure; active, systemic infection ≤14 days preceding study drug tx; and history of recurrent, invasive infections caused by encapsulated microorganisms. Primary endpoints are the proportion of pts achieving 1) a sustained increase in Hb levels from baseline (BL) of ≥2g/dL (between D126 and D168) in the absence of RBC transfusions (between D14 and D168); and 2) a sustained increase in Hb levels to ≥12g/dL (between D126 and D168), in the absence of RBC transfusions (between D14 and D168). Potential superiority of iptacopan vs ECU/RAV in the analysis of the primary endpoints will be determined using an odds ratio and tested using a conditional logistic regression model, accounting for stratification factors used at randomization and adjusted for age, sex, and BL Hb. The study α will be split equally between the two endpoints and will determine rejection of each based on the permutation distribution of the P-values. Secondary endpoints include RBC transfusion-avoidance between D14 and D168 (except D1-14); and changes from BL in Hb levels, FACIT-Fatigue score, and reticulocytes (per weighted testing approach). The primary and secondary endpoints will be tested using a graphical multiplicity adjustment, where α weights are assigned to the different tests. If all of the above hypotheses (including both primary endpoints) are rejected, the following will be tested at full study α, also in a weighted fashion: % change from BL in lactate dehydrogenase, occurrence of breakthrough hemolysis, and occurrence of major adverse vascular events (including thrombosis). Additional endpoints include safety assessments, PNH clone size, C3d deposition on RBCs, and PROs (PGI-S, EORTC QLQ-C30, EQ-5D-5L).
Results
NA
Conclusion
This study will be the first head-to-head comparison of iptacopan vs an anti-C5 MAb in patients with PNH.
Keyword(s): Anemia, Hemolysis, PNH
Abstract: PB1476
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
The standard of care in PNH is anti-C5 monoclonal antibody (MAb) treatment (tx) with eculizumab (ECU) or ravulizumab (RAV) but hematological responses can be heterogenous. Iptacopan (LNP023) is a first-in-class, oral, small-molecule, selective and reversible complement factor B inhibitor that inhibits both intra- and extravascular hemolysis. In an ongoing Ph2 study (NCT03439839), iptacopan add-on tx has demonstrated improvements in hematological response and biomarkers of disease activity in adult patients (pts) with PNH with active hemolysis despite ECU tx.
Aims
The Ph3 APPLY-PNH study (NCT04558918) will assess the efficacy and safety of iptacopan monotherapy in adult pts with PNH with residual anemia despite prior anti-C5 MAb tx by evaluating its superiority to anti-C5 MAb tx.
Methods
The study comprises an ≤8-wk screening period; a 24-wk, randomized, open‑label, active‑controlled, parallel‑group tx period; and a 24-wk tx extension period (Fig. 1). Pts (N≈91) will be randomized (8:5) to iptacopan monotherapy 200mg orally BID (seamless switch from prior tx) or intravenous ECU/RAV (continue prior tx). Pts will be stratified based on prior anti-C5 tx and transfusion history. On completion of the Wk 24 visit, pts may enter the 24-wk iptacopan tx extension period. Eligible pts must have a confirmed diagnosis of PNH (red blood cells [RBCs] and white blood cells [granulocyte/monocyte] clone size ≥10%) and residual anemia (mean Hb <10g/dL) despite receiving a stable regimen of ECU/RAV in the 6 months preceding randomization. Key exclusion criteria include ECU dosing interval ≤11 days; known or suspected hereditary complement deficiency; hematopoietic stem cell transplantation; laboratory evidence of bone marrow failure; active, systemic infection ≤14 days preceding study drug tx; and history of recurrent, invasive infections caused by encapsulated microorganisms. Primary endpoints are the proportion of pts achieving 1) a sustained increase in Hb levels from baseline (BL) of ≥2g/dL (between D126 and D168) in the absence of RBC transfusions (between D14 and D168); and 2) a sustained increase in Hb levels to ≥12g/dL (between D126 and D168), in the absence of RBC transfusions (between D14 and D168). Potential superiority of iptacopan vs ECU/RAV in the analysis of the primary endpoints will be determined using an odds ratio and tested using a conditional logistic regression model, accounting for stratification factors used at randomization and adjusted for age, sex, and BL Hb. The study α will be split equally between the two endpoints and will determine rejection of each based on the permutation distribution of the P-values. Secondary endpoints include RBC transfusion-avoidance between D14 and D168 (except D1-14); and changes from BL in Hb levels, FACIT-Fatigue score, and reticulocytes (per weighted testing approach). The primary and secondary endpoints will be tested using a graphical multiplicity adjustment, where α weights are assigned to the different tests. If all of the above hypotheses (including both primary endpoints) are rejected, the following will be tested at full study α, also in a weighted fashion: % change from BL in lactate dehydrogenase, occurrence of breakthrough hemolysis, and occurrence of major adverse vascular events (including thrombosis). Additional endpoints include safety assessments, PNH clone size, C3d deposition on RBCs, and PROs (PGI-S, EORTC QLQ-C30, EQ-5D-5L).
Results
NA

Conclusion
This study will be the first head-to-head comparison of iptacopan vs an anti-C5 MAb in patients with PNH.
Keyword(s): Anemia, Hemolysis, PNH


