
Contributions
Abstract: PB1475
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis in paroxysmal nocturnal hemoglobinuria (PNH). In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al, EHA 2020). PEG is self-administered twice weekly via two 10-mL subcutaneous injections at two distinct sites, which may lead to injection-site reactions (ISRs).
Aims
Our aim was to compare ISR safety outcomes from the PEGASUS trial to ISR incidences from similarly administered treatments based on published literature.
Methods
PEGASUS subjects received twice-weekly subcutaneous injections of 1080 mg PEG plus their current intravenous ECU dose in an initial 4-week run-in period (RIP), followed by a 16-week 1:1 randomized controlled period (RCP) where participants received PEG (n=41) or ECU monotherapy (n=39). Patients receiving ECU during the RCP entered another 4-week RIP (ECU+PEG), followed by an Open Label Period (OLP), in which all patients received PEG monotherapy.
The primary endpoint was the mean change from baseline in Hb levels at Week 16. Safety was a secondary endpoint and included monitoring incidence of ISRs, adverse events (AE), and treatment-emergent AEs (TEAEs). Therapies comparable to PEG were identified in order to establish a context for PEG-associated ISRs. Inclusion criteria were subcutaneously administered drugs with similar injection volumes (≥10 mL) or PEGylation (with ≥0.5-mL injections). ISR incidences and management strategies reported with identified drugs were evaluated from the prescribing information and published literature.
Results
Total reported TEAEs were as follows: i) RIP and open-label RIP (71 subjects, 89%); ii) RCP (PEG arm: 36 subjects [88%]; ECU arm: 36 subjects [92%]); iii) OLP (71 subjects [92%]). Most ISRs occurred during treatment initiation, as 59% of PEG patients experienced ISRs during the initial RIP compared to 37% in the RCP and 26% in the OLP. 2.6% of ECU patients experienced ISRs. In the total cohort, most ISRs were mild; there were a few moderate ISRs (4 in RIP, 3 in RCP+OLP PEG group; minimal intervention indicated) and no ISRs that were severe or led to study drug discontinuation.
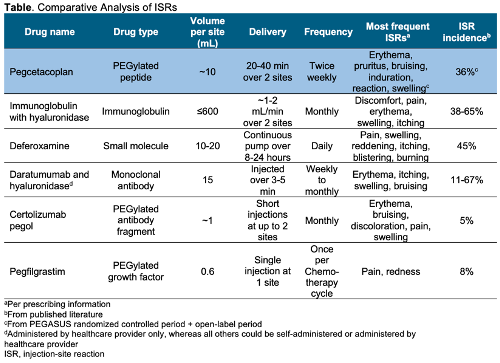
Five drugs with comparable mode of delivery to PEG were identified and evaluated for ISR data (see Table). The percent of patients who experienced ISRs for evaluated drugs varied from 5–67% but were generally mild, resolved quickly, and were less likely to occur with subsequent treatments, according to published studies. Prevention or management strategies for ISRs included ice packs, injection-site rotation, and empowering patients to gain confidence in self-administration through training and other initiatives.
Conclusion
PEGASUS trial ISRs observed with PEG treatment were more frequent in the initial treatment period and decreased over time, potentially because patients became more experienced with self-injection. ISRs were often mild or manageable and PEG patients on average reported better quality of life than ECU-treated patients at Week 16, indicating these events are likely not a barrier to treatment. Comparable trends for reported ISRs have been observed with other drugs delivered similarly to PEG; management strategies for ISRs with these drugs may potentially be useful for reactions observed with PEG.
Keyword(s): Paroxysmal nocturnal hemoglobinuria (PNH), Safety, Subcutaneous, Treatment
Abstract: PB1475
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis in paroxysmal nocturnal hemoglobinuria (PNH). In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al, EHA 2020). PEG is self-administered twice weekly via two 10-mL subcutaneous injections at two distinct sites, which may lead to injection-site reactions (ISRs).
Aims
Our aim was to compare ISR safety outcomes from the PEGASUS trial to ISR incidences from similarly administered treatments based on published literature.
Methods
PEGASUS subjects received twice-weekly subcutaneous injections of 1080 mg PEG plus their current intravenous ECU dose in an initial 4-week run-in period (RIP), followed by a 16-week 1:1 randomized controlled period (RCP) where participants received PEG (n=41) or ECU monotherapy (n=39). Patients receiving ECU during the RCP entered another 4-week RIP (ECU+PEG), followed by an Open Label Period (OLP), in which all patients received PEG monotherapy.
The primary endpoint was the mean change from baseline in Hb levels at Week 16. Safety was a secondary endpoint and included monitoring incidence of ISRs, adverse events (AE), and treatment-emergent AEs (TEAEs). Therapies comparable to PEG were identified in order to establish a context for PEG-associated ISRs. Inclusion criteria were subcutaneously administered drugs with similar injection volumes (≥10 mL) or PEGylation (with ≥0.5-mL injections). ISR incidences and management strategies reported with identified drugs were evaluated from the prescribing information and published literature.
Results
Total reported TEAEs were as follows: i) RIP and open-label RIP (71 subjects, 89%); ii) RCP (PEG arm: 36 subjects [88%]; ECU arm: 36 subjects [92%]); iii) OLP (71 subjects [92%]). Most ISRs occurred during treatment initiation, as 59% of PEG patients experienced ISRs during the initial RIP compared to 37% in the RCP and 26% in the OLP. 2.6% of ECU patients experienced ISRs. In the total cohort, most ISRs were mild; there were a few moderate ISRs (4 in RIP, 3 in RCP+OLP PEG group; minimal intervention indicated) and no ISRs that were severe or led to study drug discontinuation.
Five drugs with comparable mode of delivery to PEG were identified and evaluated for ISR data (see Table). The percent of patients who experienced ISRs for evaluated drugs varied from 5–67% but were generally mild, resolved quickly, and were less likely to occur with subsequent treatments, according to published studies. Prevention or management strategies for ISRs included ice packs, injection-site rotation, and empowering patients to gain confidence in self-administration through training and other initiatives.

Conclusion
PEGASUS trial ISRs observed with PEG treatment were more frequent in the initial treatment period and decreased over time, potentially because patients became more experienced with self-injection. ISRs were often mild or manageable and PEG patients on average reported better quality of life than ECU-treated patients at Week 16, indicating these events are likely not a barrier to treatment. Comparable trends for reported ISRs have been observed with other drugs delivered similarly to PEG; management strategies for ISRs with these drugs may potentially be useful for reactions observed with PEG.
Keyword(s): Paroxysmal nocturnal hemoglobinuria (PNH), Safety, Subcutaneous, Treatment


