
Contributions
Abstract: PB1471
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al, EHA 2020).
Aims
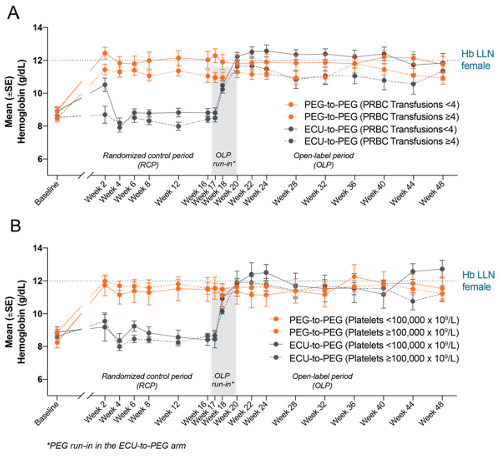
To identify if the prespecified strata (transfusion history and platelet count) of PEGASUS patients showed additional benefit from PEG vs ECU at 48 weeks.
Methods
Eighty PNH patients (≥18 years) with Hb levels <10.5 g/dL and ECU treatment (≥3 months) were enrolled. Patients entered a 4-week run-in period (RIP) with ECU+PEG (1080 mg subcutaneously 2x/week), then were stratified based on baseline platelet count and prior transfusion requirements, and randomized 1:1 to PEG or ECU monotherapy. The primary endpoint was change in Hb level from baseline to Week 16. Patients could continue to an open label period (OLP) of PEG monotherapy, which included a 4-week RIP (ECU+PEG) for ECU patients (ECU-to-PEG) but not for patients continuing PEG (PEG-to-PEG). Key secondary endpoints were transfusion avoidance and adverse events (AEs). Here, primary and key secondary endpoints were analyzed by subgroups (<4 vs ≥4 packed red blood cell transfusions within 12 months prior to baseline; platelet count at screening [<100,000x109/L vs ≥100,000x109/L]).
Results
PEG treatment was associated with significantly greater increases in Hb levels from baseline than ECU at Week 16, which were maintained in the PEG-to-PEG arm through Week 48 regardless of baseline subgroup. The ECU-to-PEG arm demonstrated increased Hb levels regardless of prior transfusions (Figure A) or baseline platelet count (Figure B). At Week 48, Hb levels were similar in the <4 (mean [SD]; PEG-to-PEG: 11.8 [1.9] g/dL; ECU-to-PEG: 11.8 [2.2] g/dL) and ≥4 transfusion strata (PEG-to-PEG: 10.9 [1.6] g/dL; ECU-to-PEG: 11.4 [2.2] g/dL). Similar Hb levels were also seen for the <100,000x109/L (PEG-to-PEG: 11.5 [2.2] g/dL; ECU-to-PEG: 12.7 [1.4] g/dL) and ≥100,000x109/L platelet strata (PEG-to-PEG: 11.2 [1.6] g/dL; ECU-to-PEG: 11.2 [2.3] g/dL).
At Week 48, on PEG monotherapy, both arms had similar proportions of transfusion free patients during the OLP in the <4 (PEG-to-PEG: 80%; ECU-to-PEG: 88%) and ≥4 transfusion strata (PEG-to-PEG: 67%; ECU-to-PEG: 61%). Similar trends were seen in the <100,000x109/L (PEG-to-PEG: 75%; ECU-to-PEG: 78%) and ≥100,000x109/L platelet strata (PEG-to-PEG: 72%; ECU-to-PEG: 70%).
The most common AEs by physician reported preferred term throughout the study for all patients who received PEG were injection site reactions (36%), hemolysis (24%), and diarrhea (21%). Of all study patients, 30% experienced serious AEs, 6% possibly related to PEG. No cases of meningitis were reported. One COVID-19 death was reported, unrelated to study treatment. Six patients discontinued due to hemolytic events: 5 classified by the treating physician as “hemolysis” and 1 as “hemolytic anemia.” Overall, 12 patients (15%) discontinued PEG: 3 in RCP, 8 in OLP due to TEAEs (6 in ECU-to-PEG, 2 in PEG-to-PEG), and one during follow-up; one patient withdrew due to physician decision.
Conclusion
In the prespecified stratified analysis of PEGASUS, PEG showed treatment effect durability in Hb level with transfusion avoidance in most PNH patients regardless of prior transfusions or baseline platelet count during the OLP through Week 48. The safety profile of PEG was consistent with previously reported data.
Keyword(s): Clinical trial, Hemoglobin, Paroxysmal nocturnal hemoglobinuria (PNH), Transfusion
Abstract: PB1471
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Clinical
Background
Pegcetacoplan (PEG), a PEGylated peptide targeting proximal complement protein C3, can control both intravascular and extravascular hemolysis. In the PEGASUS trial (NCT03500549), a phase 3, randomized, open-label, active-comparator controlled study, PEG was shown to be superior to eculizumab (ECU) after 16 weeks in improving hemoglobin (Hb) levels and clinical outcomes in patients with PNH (Hillmen P et al, EHA 2020).
Aims
To identify if the prespecified strata (transfusion history and platelet count) of PEGASUS patients showed additional benefit from PEG vs ECU at 48 weeks.
Methods
Eighty PNH patients (≥18 years) with Hb levels <10.5 g/dL and ECU treatment (≥3 months) were enrolled. Patients entered a 4-week run-in period (RIP) with ECU+PEG (1080 mg subcutaneously 2x/week), then were stratified based on baseline platelet count and prior transfusion requirements, and randomized 1:1 to PEG or ECU monotherapy. The primary endpoint was change in Hb level from baseline to Week 16. Patients could continue to an open label period (OLP) of PEG monotherapy, which included a 4-week RIP (ECU+PEG) for ECU patients (ECU-to-PEG) but not for patients continuing PEG (PEG-to-PEG). Key secondary endpoints were transfusion avoidance and adverse events (AEs). Here, primary and key secondary endpoints were analyzed by subgroups (<4 vs ≥4 packed red blood cell transfusions within 12 months prior to baseline; platelet count at screening [<100,000x109/L vs ≥100,000x109/L]).
Results
PEG treatment was associated with significantly greater increases in Hb levels from baseline than ECU at Week 16, which were maintained in the PEG-to-PEG arm through Week 48 regardless of baseline subgroup. The ECU-to-PEG arm demonstrated increased Hb levels regardless of prior transfusions (Figure A) or baseline platelet count (Figure B). At Week 48, Hb levels were similar in the <4 (mean [SD]; PEG-to-PEG: 11.8 [1.9] g/dL; ECU-to-PEG: 11.8 [2.2] g/dL) and ≥4 transfusion strata (PEG-to-PEG: 10.9 [1.6] g/dL; ECU-to-PEG: 11.4 [2.2] g/dL). Similar Hb levels were also seen for the <100,000x109/L (PEG-to-PEG: 11.5 [2.2] g/dL; ECU-to-PEG: 12.7 [1.4] g/dL) and ≥100,000x109/L platelet strata (PEG-to-PEG: 11.2 [1.6] g/dL; ECU-to-PEG: 11.2 [2.3] g/dL).
At Week 48, on PEG monotherapy, both arms had similar proportions of transfusion free patients during the OLP in the <4 (PEG-to-PEG: 80%; ECU-to-PEG: 88%) and ≥4 transfusion strata (PEG-to-PEG: 67%; ECU-to-PEG: 61%). Similar trends were seen in the <100,000x109/L (PEG-to-PEG: 75%; ECU-to-PEG: 78%) and ≥100,000x109/L platelet strata (PEG-to-PEG: 72%; ECU-to-PEG: 70%).
The most common AEs by physician reported preferred term throughout the study for all patients who received PEG were injection site reactions (36%), hemolysis (24%), and diarrhea (21%). Of all study patients, 30% experienced serious AEs, 6% possibly related to PEG. No cases of meningitis were reported. One COVID-19 death was reported, unrelated to study treatment. Six patients discontinued due to hemolytic events: 5 classified by the treating physician as “hemolysis” and 1 as “hemolytic anemia.” Overall, 12 patients (15%) discontinued PEG: 3 in RCP, 8 in OLP due to TEAEs (6 in ECU-to-PEG, 2 in PEG-to-PEG), and one during follow-up; one patient withdrew due to physician decision.

Conclusion
In the prespecified stratified analysis of PEGASUS, PEG showed treatment effect durability in Hb level with transfusion avoidance in most PNH patients regardless of prior transfusions or baseline platelet count during the OLP through Week 48. The safety profile of PEG was consistent with previously reported data.
Keyword(s): Clinical trial, Hemoglobin, Paroxysmal nocturnal hemoglobinuria (PNH), Transfusion


