
Contributions
Abstract: PB1467
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Biology & Translational Research
Background
Aplastic anemia (AA) is caused by hematopoietic stem cells (HSC) damage resulting in a bone marrow (BM) failure. An autoimmune attack is the most frequent but probably not the only origin of it.
Aims
The aim of the study was to analyze stromal microenvironment in BM of untreated AA patients through the multipotent mesenchymal stromal cells (MMSC).
Methods
The study included 21 AA (8 with severe AA (SAA) and 13 with non-severe AA (NAA)) patients in the debut. In all patients BM was aspirated after informed consent at diagnostic punctures. MMSC were isolated and cultivated by the standard method. RNA was extracted on passage 1 by the standard protocol. Gene expression was analyzed with Taqman modification of RT-PCR. As a control BM samples of 19 healthy donors of according age were used.
Results
Comparison of gene expression in MMSC from SAA and NAA patients revealed the significant decrease in the expression of FGF2, SDF1, and IL1R in SAA patients. The expression of FGFR2, PTGES, and ANGPT1 was significantly decreased in SAA patients compared to donors. MMSCs from NAA patients, on the contrary, revealed increased expression of FGFR1, TGFB2, and CFH in comparison with donors. Thus, stromal precursors in NAA and SAA are altered differently. Probably, it reflects initial differences in the pathogenesis of these forms of AA.
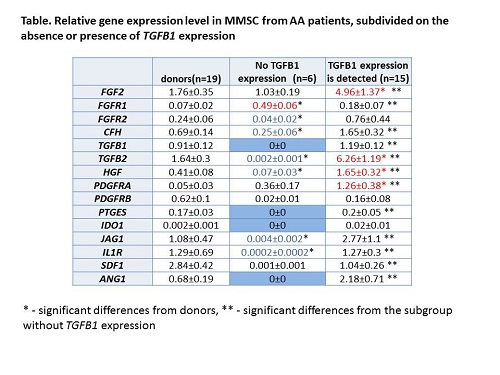
In MMSC of 6 AA patients (3 with NAA and 3 with SAA) there was no expression of TGFB1, ANGPT1, IDO1, and PTGES. Moreover most of the other studied genes were dramatically down-regulated in these MMSC (Table). In 5 out of 6 patients these changes were detected even after 6 months of immunosuppressive therapy, while all these patients had responded to the treatment and reached partial remission or hematological improvement within 6 months. So the disrupted expression of these genes may be permanent. Despite of the absence of expression TGFB1 and ANGPT1, which are factor crucial for the hematopoiesis, the hematopoiesis was partly restored. Both TGFB1 and ANGPT1 regulate quiescence of HSC. Therefore, the lack of these factors could induce HSC activation. The determination of these factors expression in long-term observation may explain mechanisms of HSC survival in such patients. What factors are responsible for the maintenance of hematopoiesis in these patients is the subject of ongoing study.
Conclusion
Stromal precursors in BM of untreated NAA and SAA patients are impaired differently. This effect could reflect the different causes of these forms of AA or be the consequence of compensatory reaction of stromal microenvironment to the various levels of hematopoiesis failure. Two subgroups of AA patients had been revealed, which dramatically differ in the pattern of gene expression in MMSCs.
Keyword(s): Aplastic anemia, Bone marrow failure, Bone marrow stroma, Mesenchymal stem cell
Abstract: PB1467
Type: Publication Only
Session title: Bone marrow failure syndromes incl. PNH - Biology & Translational Research
Background
Aplastic anemia (AA) is caused by hematopoietic stem cells (HSC) damage resulting in a bone marrow (BM) failure. An autoimmune attack is the most frequent but probably not the only origin of it.
Aims
The aim of the study was to analyze stromal microenvironment in BM of untreated AA patients through the multipotent mesenchymal stromal cells (MMSC).
Methods
The study included 21 AA (8 with severe AA (SAA) and 13 with non-severe AA (NAA)) patients in the debut. In all patients BM was aspirated after informed consent at diagnostic punctures. MMSC were isolated and cultivated by the standard method. RNA was extracted on passage 1 by the standard protocol. Gene expression was analyzed with Taqman modification of RT-PCR. As a control BM samples of 19 healthy donors of according age were used.
Results
Comparison of gene expression in MMSC from SAA and NAA patients revealed the significant decrease in the expression of FGF2, SDF1, and IL1R in SAA patients. The expression of FGFR2, PTGES, and ANGPT1 was significantly decreased in SAA patients compared to donors. MMSCs from NAA patients, on the contrary, revealed increased expression of FGFR1, TGFB2, and CFH in comparison with donors. Thus, stromal precursors in NAA and SAA are altered differently. Probably, it reflects initial differences in the pathogenesis of these forms of AA.
In MMSC of 6 AA patients (3 with NAA and 3 with SAA) there was no expression of TGFB1, ANGPT1, IDO1, and PTGES. Moreover most of the other studied genes were dramatically down-regulated in these MMSC (Table). In 5 out of 6 patients these changes were detected even after 6 months of immunosuppressive therapy, while all these patients had responded to the treatment and reached partial remission or hematological improvement within 6 months. So the disrupted expression of these genes may be permanent. Despite of the absence of expression TGFB1 and ANGPT1, which are factor crucial for the hematopoiesis, the hematopoiesis was partly restored. Both TGFB1 and ANGPT1 regulate quiescence of HSC. Therefore, the lack of these factors could induce HSC activation. The determination of these factors expression in long-term observation may explain mechanisms of HSC survival in such patients. What factors are responsible for the maintenance of hematopoiesis in these patients is the subject of ongoing study.

Conclusion
Stromal precursors in BM of untreated NAA and SAA patients are impaired differently. This effect could reflect the different causes of these forms of AA or be the consequence of compensatory reaction of stromal microenvironment to the various levels of hematopoiesis failure. Two subgroups of AA patients had been revealed, which dramatically differ in the pattern of gene expression in MMSCs.
Keyword(s): Aplastic anemia, Bone marrow failure, Bone marrow stroma, Mesenchymal stem cell


