
Contributions
Abstract: PB1451
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is an Fc-modified, humanized monoclonal antibody targeting CD19+ tumor cells, which enhances in vitro antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis. Lenalidomide (LEN) has anti-proliferative and anti-angiogenic activities. Tafasitamab + LEN is indicated in adult patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including arising from low-grade lymphoma, who are ineligible for autologous stem cell transplantation. The L-MIND study (NCT02399085) showed that tafasitamab + LEN is well tolerated, with an ORR of 57.5% at ≥35 months f/up (reported at this meeting) in pts with R/R DLBCL. R-CHOP is the current standard of care for pts with newly-diagnosed DLBCL. In preclinical studies, tafasitamab + rituximab and tafasitamab + LEN were shown to increase anti-tumor activity compared to either single agent alone. Data from FIRST-MIND (NCT04134936), a Phase Ib, open‑label, randomized study of tafasitamab + R-CHOP or tafasitamab + LEN + R‑CHOP in pts with newly-diagnosed DLBCL, are reported elsewhere at this congress.
Aims
The Front-MIND study aims to assess the efficacy and safety of tafasitamab + LEN + R-CHOP vs R-CHOP alone in previously untreated, high-intermediate and high-risk pts.
Methods
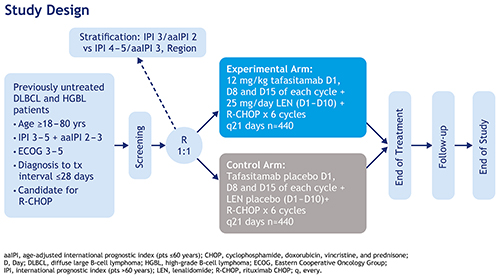
Front-MIND is a Phase III, multicenter, randomized, double-blind, placebo-controlled study (Fig.). An estimated 880 pts will be enrolled, from 350 centers in America, Europe and Asia-Pacific. Eligible pts are aged 18 to 80 yrs with previously untreated (diagnosis to start of treatment ≤28 days [D]) local biopsy-proven, CD20-positive DLBCL (International Prognostic Index [IPI] ≥3 if >60 yrs/age-adjusted IPI 2–3 if ≤60 yrs), and ECOG PS 0–2. Pts with transformed lymphoma are excluded. Pts will be randomized 1:1 based on stratification factors: IPI intermediate-high vs high risk and geographic region. The experimental arm will comprise six 21-D cycles of tafasitamab (12 mg/kg intravenous D 1, 8 and 15) + LEN (25 mg orally, D1–10) + R-CHOP. The control arm will comprise six 21-D cycles of tafasitamab placebo (0.9% saline solution IV, D1, 8 and 15) + LEN placebo (D1–10) + R‑CHOP. Pre-planned Central Nervous System prophylaxis with intravenous high-dose methotrexate is allowed after response assessment at end of treatment. The primary endpoint is investigator-assessed progression-free survival. Secondary endpoints include investigator-assessed event-free survival and overall survival. A large set of translational studies is planned, analyzing the relationship between potential biomarkers and efficacy. Pts will be followed up from their end-of-treatment visit until the end of the study, including clinical evaluation every 3–6 months and imaging every 3–12 months up to 60 months. The expected study duration from first pt’s first visit to last pt’s last visit is approximately 5 years. The study is conducted with the scientific support of the Fondazione Italiana Linfomi and the German Lymphoma Alliance.
Results
Results for this study are not yet available.
Conclusion
There remains a high unmet need to improve treatment options for newly diagnosed pts with high-risk DLBCL. The combination of tafasitamab, LEN and R-CHOP may have synergistic potential. Preliminary data from the First-MIND study suggest that tafasitamab ± LEN + R-CHOP is tolerable in pts with treatment‑naïve DLBCL. The Phase III Front-MIND study will provide further evaluation of clinical benefits and safety in a high-intermediate and high-risk population of pts with newly diagnosed DLBCL.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody
Abstract: PB1451
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is an Fc-modified, humanized monoclonal antibody targeting CD19+ tumor cells, which enhances in vitro antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis. Lenalidomide (LEN) has anti-proliferative and anti-angiogenic activities. Tafasitamab + LEN is indicated in adult patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including arising from low-grade lymphoma, who are ineligible for autologous stem cell transplantation. The L-MIND study (NCT02399085) showed that tafasitamab + LEN is well tolerated, with an ORR of 57.5% at ≥35 months f/up (reported at this meeting) in pts with R/R DLBCL. R-CHOP is the current standard of care for pts with newly-diagnosed DLBCL. In preclinical studies, tafasitamab + rituximab and tafasitamab + LEN were shown to increase anti-tumor activity compared to either single agent alone. Data from FIRST-MIND (NCT04134936), a Phase Ib, open‑label, randomized study of tafasitamab + R-CHOP or tafasitamab + LEN + R‑CHOP in pts with newly-diagnosed DLBCL, are reported elsewhere at this congress.
Aims
The Front-MIND study aims to assess the efficacy and safety of tafasitamab + LEN + R-CHOP vs R-CHOP alone in previously untreated, high-intermediate and high-risk pts.
Methods
Front-MIND is a Phase III, multicenter, randomized, double-blind, placebo-controlled study (Fig.). An estimated 880 pts will be enrolled, from 350 centers in America, Europe and Asia-Pacific. Eligible pts are aged 18 to 80 yrs with previously untreated (diagnosis to start of treatment ≤28 days [D]) local biopsy-proven, CD20-positive DLBCL (International Prognostic Index [IPI] ≥3 if >60 yrs/age-adjusted IPI 2–3 if ≤60 yrs), and ECOG PS 0–2. Pts with transformed lymphoma are excluded. Pts will be randomized 1:1 based on stratification factors: IPI intermediate-high vs high risk and geographic region. The experimental arm will comprise six 21-D cycles of tafasitamab (12 mg/kg intravenous D 1, 8 and 15) + LEN (25 mg orally, D1–10) + R-CHOP. The control arm will comprise six 21-D cycles of tafasitamab placebo (0.9% saline solution IV, D1, 8 and 15) + LEN placebo (D1–10) + R‑CHOP. Pre-planned Central Nervous System prophylaxis with intravenous high-dose methotrexate is allowed after response assessment at end of treatment. The primary endpoint is investigator-assessed progression-free survival. Secondary endpoints include investigator-assessed event-free survival and overall survival. A large set of translational studies is planned, analyzing the relationship between potential biomarkers and efficacy. Pts will be followed up from their end-of-treatment visit until the end of the study, including clinical evaluation every 3–6 months and imaging every 3–12 months up to 60 months. The expected study duration from first pt’s first visit to last pt’s last visit is approximately 5 years. The study is conducted with the scientific support of the Fondazione Italiana Linfomi and the German Lymphoma Alliance.
Results
Results for this study are not yet available.

Conclusion
There remains a high unmet need to improve treatment options for newly diagnosed pts with high-risk DLBCL. The combination of tafasitamab, LEN and R-CHOP may have synergistic potential. Preliminary data from the First-MIND study suggest that tafasitamab ± LEN + R-CHOP is tolerable in pts with treatment‑naïve DLBCL. The Phase III Front-MIND study will provide further evaluation of clinical benefits and safety in a high-intermediate and high-risk population of pts with newly diagnosed DLBCL.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody


