
Contributions
Abstract: PB1450
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The standard first-line therapy for diffuse large B-cell lymphoma (DLBCL) is CHOP-R. Many studies have shown the efficacy of EDOCH-R (etoposide, dexamethasone, vincristine, cyclophosphamide, doxorubicin and rituximab) regimen in this pathology with an acceptable toxicity. However, there is few evidence supporting the efficacy and safety in elder patients.
Aims
The aim of this study is to analyze the results of EDOCH-R as first line therapy in patients over 60 years old with de novo DLBCL diagnosis at Hospital Universitario de Guadalajara (HUG).
Methods
The medical records of all the patients with DLBCL over the age of 60, treated with EDOCH-R as first-line therapy at HUG from 01/01/2014 to 31/12/2020 were review, identifying 25 patients in total.
Baseline data was collected at diagnosis, including patient demographics, Eastern Cooperative Oncology Group (ECOG), staging, IPI, PET-TC and histologic subtypes. Germinal center B-cell (GCB) and non-GCB DLBCL subtypes were classified based on the algorithm proposed by Hans et al. EDOCH-R administration was planned every 21 days for 6 cycles. None of the patients received radiotherapy. Pegfilgrastim was administered at day 6 of therapy. Adverse events were reported according to the National Cancer Institute reviewed criteria. Statistical analysis was carried out with SPSS software. Overall survival (OS), progression-free survival (PFS) and response rate were the primary end points of this study. The secondary clinical objective included patient safety.
Results
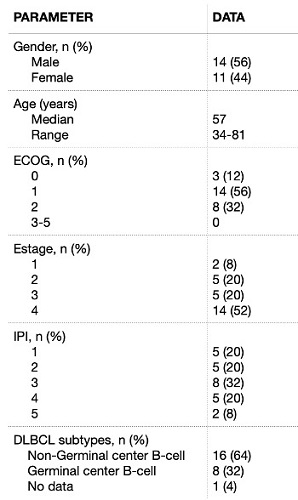
The diagnostic parameters obtained for all the patients are recorded in Table 1. All the patients exhibited a high Ki-67 expression (≥ 80%). Two patients (8%) patients were classified as double expressor (DE) and another 2 (8%) individuals resulted double-hit lymphomas (DHL).
All the patients received EDOCH-R regimen as first-line chemotherapy with a median of 6 cycles. Complete remission (CR) was achieved in 79% of the patients that completed the 6 cycles, and partial remission was achieved in 10% of the patients. One patient (5%) exhibited progressive disease after 4 cycles of therapy. With a median follow-up of 36 months the OS was 65% and PFS was 52%. OS was lower in the group of patients older than 70 years and in those with an IPI greater than 3, although a significant difference was not encountered.
The major side effect of the EDOCH-R regimen was hematologic toxicity. Grade 4 neutropenia, anemia and thrombocytopenia were observed in 76%, 36% and 24% of the patients, respectively. Neutropenic fever developed in 76% of the patients. One patient exhibited a decrease in the cardiac ejection fraction, which led to change the treatment. Three patients (12%) over 70-years-old died of progressive lymphoma, and 2 patients (8%) had to end therapy due to comorbidity factors.
Conclusion
EDOCH-R is an effective therapy for DLBCL in elderly patients, although it has shown a high hematological toxicity.
Keyword(s): Diffuse large B cell lymphoma, DLBCL, Etoposide, Survival
Abstract: PB1450
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
The standard first-line therapy for diffuse large B-cell lymphoma (DLBCL) is CHOP-R. Many studies have shown the efficacy of EDOCH-R (etoposide, dexamethasone, vincristine, cyclophosphamide, doxorubicin and rituximab) regimen in this pathology with an acceptable toxicity. However, there is few evidence supporting the efficacy and safety in elder patients.
Aims
The aim of this study is to analyze the results of EDOCH-R as first line therapy in patients over 60 years old with de novo DLBCL diagnosis at Hospital Universitario de Guadalajara (HUG).
Methods
The medical records of all the patients with DLBCL over the age of 60, treated with EDOCH-R as first-line therapy at HUG from 01/01/2014 to 31/12/2020 were review, identifying 25 patients in total.
Baseline data was collected at diagnosis, including patient demographics, Eastern Cooperative Oncology Group (ECOG), staging, IPI, PET-TC and histologic subtypes. Germinal center B-cell (GCB) and non-GCB DLBCL subtypes were classified based on the algorithm proposed by Hans et al. EDOCH-R administration was planned every 21 days for 6 cycles. None of the patients received radiotherapy. Pegfilgrastim was administered at day 6 of therapy. Adverse events were reported according to the National Cancer Institute reviewed criteria. Statistical analysis was carried out with SPSS software. Overall survival (OS), progression-free survival (PFS) and response rate were the primary end points of this study. The secondary clinical objective included patient safety.
Results
The diagnostic parameters obtained for all the patients are recorded in Table 1. All the patients exhibited a high Ki-67 expression (≥ 80%). Two patients (8%) patients were classified as double expressor (DE) and another 2 (8%) individuals resulted double-hit lymphomas (DHL).
All the patients received EDOCH-R regimen as first-line chemotherapy with a median of 6 cycles. Complete remission (CR) was achieved in 79% of the patients that completed the 6 cycles, and partial remission was achieved in 10% of the patients. One patient (5%) exhibited progressive disease after 4 cycles of therapy. With a median follow-up of 36 months the OS was 65% and PFS was 52%. OS was lower in the group of patients older than 70 years and in those with an IPI greater than 3, although a significant difference was not encountered.
The major side effect of the EDOCH-R regimen was hematologic toxicity. Grade 4 neutropenia, anemia and thrombocytopenia were observed in 76%, 36% and 24% of the patients, respectively. Neutropenic fever developed in 76% of the patients. One patient exhibited a decrease in the cardiac ejection fraction, which led to change the treatment. Three patients (12%) over 70-years-old died of progressive lymphoma, and 2 patients (8%) had to end therapy due to comorbidity factors.

Conclusion
EDOCH-R is an effective therapy for DLBCL in elderly patients, although it has shown a high hematological toxicity.
Keyword(s): Diffuse large B cell lymphoma, DLBCL, Etoposide, Survival


