
Contributions
Abstract: PB1444
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is a humanized, Fc-modified anti-CD19 monoclonal antibody that enhances antibody‑dependent cellular cytotoxicity and phagocytosis. It is FDA-approved in combination with lenalidomide for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from low‑grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT).
Aims
L-MIND (NCT02399085) is an ongoing, open-label, single-arm, Phase II study to characterize the safety and efficacy of tafasitamab + lenalidomide in ASCT-ineligible patients with R/R DLBCL. Primary analyses and 2-year efficacy results were previously presented: we report an updated efficacy analysis with ≥35 months follow up (Data cut-off: October 30, 2020).
Methods
Patients were aged ≥18 years with ASCT-ineligible R/R DLBCL, had 1–3 prior systemic therapies (including ≥1 CD20-targeting regimen), with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients received 28-day cycles of tafasitamab (12 mg/kg IV), once weekly during cycles 1–3, with a loading dose on Day 4 of cycle 1, then every two weeks (Q2W) during cycles 4–12. Lenalidomide (25 mg PO) was administered on Days 1–21 of cycles 1–12. After cycle 12, progression-free patients received tafasitamab Q2W until disease progression. The primary endpoint was objective response rate (ORR), assessed by IRC. Secondary endpoints included duration of response (DoR), progression-free survival (PFS) and overall survival (OS).
Results
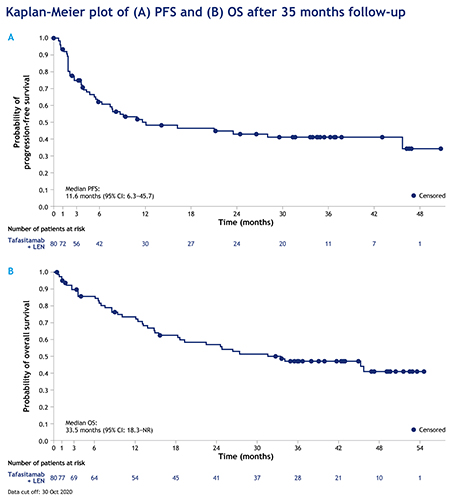
Eighty of 81 enrolled patients received tafasitamab + lenalidomide and were included in the full analysis set. At data cut-off, the overall ORR was 57.5% (n=46/80), including a complete response (CR) in 40% of patients (n=32/80) and a partial response (PR) in 17.5% of patients (n=14/80). Median estimated DoR was 43.9 months (95% confidence interval (CI): 26.1–not reached [NR]), and was NR in patients who achieved a CR (95% CI: 43.9–NR). Median estimated PFS was 11.6 months (95% CI: 6.3–45.7), with a median follow-up of 33.9 months (Fig.); estimated 36-month PFS was 41.1% (95% CI: 29.1–52.7). Median estimated OS was 33.5 months (95% CI: 18.3–NR), with a median follow-up of 42.7 months.
In patients who had received 1 vs ≥2 prior lines of therapy, ORR was 67.5% (n=27/40; CR rate: 47.5%; PR rate: 20.0%) vs 47.5% (n=19/40; CR rate: 32.5%; PR rate: 15.0%). Median estimated DoR was 43.9 months (95% CI 9.1–NR) in patients with 1 prior treatment and not reached (95% CI 15.0–NR) in those receiving ≥2 prior treatments. Median estimated PFS and estimated 36-month PFS were 23.5 months (95% CI 7.4–NR) and 47.8% (95% CI: 30.2–63.4) in patients with 1 prior treatment vs 7.6 months (95% CI 2.7–NR) and 34.3% (95% CI: 18.6–50.7) in those receiving ≥2 prior treatments. Median estimated OS was 45.7 months (95% CI 24.6–NR) in patients with 1 prior treatment compared with 15.5 months (95% CI 8.6–NR) in those receiving ≥2 prior treatments.
There were no unexpected toxicities or new safety signals.
Conclusion
Combination therapy with tafasitamab + lenalidomide followed by tafasitamab monotherapy provided durable responses, achieving a median DoR of 43.9 months in patients with R/R DLBCL not eligible for ASCT, with a manageable safety profile. These long-term data indicate that this chemotherapy-free regimen can achieve prolonged remission and survival benefit in this patient population, especially at first relapse, with a median estimated DoR of 43.9 months and median estimated OS of 45.7 months at second-line.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody
Abstract: PB1444
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Tafasitamab is a humanized, Fc-modified anti-CD19 monoclonal antibody that enhances antibody‑dependent cellular cytotoxicity and phagocytosis. It is FDA-approved in combination with lenalidomide for adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), including arising from low‑grade lymphoma, who are ineligible for autologous stem cell transplant (ASCT).
Aims
L-MIND (NCT02399085) is an ongoing, open-label, single-arm, Phase II study to characterize the safety and efficacy of tafasitamab + lenalidomide in ASCT-ineligible patients with R/R DLBCL. Primary analyses and 2-year efficacy results were previously presented: we report an updated efficacy analysis with ≥35 months follow up (Data cut-off: October 30, 2020).
Methods
Patients were aged ≥18 years with ASCT-ineligible R/R DLBCL, had 1–3 prior systemic therapies (including ≥1 CD20-targeting regimen), with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients received 28-day cycles of tafasitamab (12 mg/kg IV), once weekly during cycles 1–3, with a loading dose on Day 4 of cycle 1, then every two weeks (Q2W) during cycles 4–12. Lenalidomide (25 mg PO) was administered on Days 1–21 of cycles 1–12. After cycle 12, progression-free patients received tafasitamab Q2W until disease progression. The primary endpoint was objective response rate (ORR), assessed by IRC. Secondary endpoints included duration of response (DoR), progression-free survival (PFS) and overall survival (OS).
Results
Eighty of 81 enrolled patients received tafasitamab + lenalidomide and were included in the full analysis set. At data cut-off, the overall ORR was 57.5% (n=46/80), including a complete response (CR) in 40% of patients (n=32/80) and a partial response (PR) in 17.5% of patients (n=14/80). Median estimated DoR was 43.9 months (95% confidence interval (CI): 26.1–not reached [NR]), and was NR in patients who achieved a CR (95% CI: 43.9–NR). Median estimated PFS was 11.6 months (95% CI: 6.3–45.7), with a median follow-up of 33.9 months (Fig.); estimated 36-month PFS was 41.1% (95% CI: 29.1–52.7). Median estimated OS was 33.5 months (95% CI: 18.3–NR), with a median follow-up of 42.7 months.
In patients who had received 1 vs ≥2 prior lines of therapy, ORR was 67.5% (n=27/40; CR rate: 47.5%; PR rate: 20.0%) vs 47.5% (n=19/40; CR rate: 32.5%; PR rate: 15.0%). Median estimated DoR was 43.9 months (95% CI 9.1–NR) in patients with 1 prior treatment and not reached (95% CI 15.0–NR) in those receiving ≥2 prior treatments. Median estimated PFS and estimated 36-month PFS were 23.5 months (95% CI 7.4–NR) and 47.8% (95% CI: 30.2–63.4) in patients with 1 prior treatment vs 7.6 months (95% CI 2.7–NR) and 34.3% (95% CI: 18.6–50.7) in those receiving ≥2 prior treatments. Median estimated OS was 45.7 months (95% CI 24.6–NR) in patients with 1 prior treatment compared with 15.5 months (95% CI 8.6–NR) in those receiving ≥2 prior treatments.
There were no unexpected toxicities or new safety signals.

Conclusion
Combination therapy with tafasitamab + lenalidomide followed by tafasitamab monotherapy provided durable responses, achieving a median DoR of 43.9 months in patients with R/R DLBCL not eligible for ASCT, with a manageable safety profile. These long-term data indicate that this chemotherapy-free regimen can achieve prolonged remission and survival benefit in this patient population, especially at first relapse, with a median estimated DoR of 43.9 months and median estimated OS of 45.7 months at second-line.
Keyword(s): CD19, DLBCL, Lymphoma, Monoclonal antibody


