Contributions
Abstract: PB1442
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Primary diffuse B-cell lymphoma of central nervous system (PCNSL) is a rare and aggressive non Hodgkin Lymphoma (2-3% of NHL). Currently, the standard of care for young and fit patients (pts), is MATRix regimen (Rituximab, HD-Metotrexate, Cytarabine and Thiothepa) and consolidation therapy with autologous stem cell transplantation (ASCT). The mortality reported in literature is 4-6%.
Aims
To study the safety and tolerability of MATRix regimen with sequential autologous peripheral blood stem cell (PBSC) infusion in young and fit pts with PCNSL.
Methods
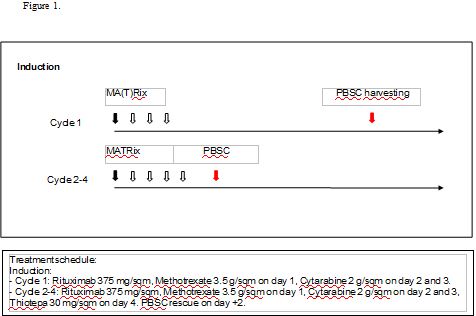
We report 10 sequential cases of young (< 70 years old) and fit pts with a PCNSL, treated at Niguarda Hospital with the first cycle of MA(T)Rix followed by PBSC harvesting and 3 MATRix with PBSC rescue. Consolidation with ASCT (thiothepa and BCNU conditioning regimen) was performed in all the population in CR/PR.
Results
Ten pts with new diagnosis of PCNSL were treated between October 2014 and March 2019. The population was composed of 8 women and 2 men, median age at diagnosis was 56 years old. Consolidation therapy with high-dose chemotherapy (HDT) and ASCT was performed in 9/10 pts. One received only radiotherapy for progressive disease (PD).
After the first cycle of chemotherapy all pts had a successful stem cell mobilization and adequate harvesting of hematopoietic progenitor cells (median 33.4 x10^6 CD34/Kg). All the following 3 cycles of MATRix had received a rescue of stem cells (median of PBSC infusion at each cycle: 5.3 x10^6 CD34/Kg) on day + 2.
The median rise of neutrophil count (N >1000/mcl) was 13 days after second cycle and 14 days after third and fourth cycles.
Regarding toxicity the major complication were infections. 10/19 (52%) of all the infections occurred during the first cycle. According to NCCN Guidelines ® we observed 15 (79%) grade 1 and 2 infections and 4 (21%) grade 3 or 4.
Neither TRM nor ICU transferral were observed. No patient experienced cardiac or thromboembolic complication. Despite the higher frequency of complication of the first cycle no dose adjustment were made on the following cycles.
With a median of follow up of 16.1 months 50% of pts are in CR, the median OS is 13.6 months and the median PFS is 9 months.
Conclusion
With the limitation of a small series of cases, MATRix regimen with sequential infusion of PBSC and ASCT consolidation is feasible and safe.
Keyword(s): Autologous hematopoietic stem cell transplantation, CNS lymphoma, NHL
Abstract: PB1442
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Primary diffuse B-cell lymphoma of central nervous system (PCNSL) is a rare and aggressive non Hodgkin Lymphoma (2-3% of NHL). Currently, the standard of care for young and fit patients (pts), is MATRix regimen (Rituximab, HD-Metotrexate, Cytarabine and Thiothepa) and consolidation therapy with autologous stem cell transplantation (ASCT). The mortality reported in literature is 4-6%.
Aims
To study the safety and tolerability of MATRix regimen with sequential autologous peripheral blood stem cell (PBSC) infusion in young and fit pts with PCNSL.
Methods
We report 10 sequential cases of young (< 70 years old) and fit pts with a PCNSL, treated at Niguarda Hospital with the first cycle of MA(T)Rix followed by PBSC harvesting and 3 MATRix with PBSC rescue. Consolidation with ASCT (thiothepa and BCNU conditioning regimen) was performed in all the population in CR/PR.
Results
Ten pts with new diagnosis of PCNSL were treated between October 2014 and March 2019. The population was composed of 8 women and 2 men, median age at diagnosis was 56 years old. Consolidation therapy with high-dose chemotherapy (HDT) and ASCT was performed in 9/10 pts. One received only radiotherapy for progressive disease (PD).
After the first cycle of chemotherapy all pts had a successful stem cell mobilization and adequate harvesting of hematopoietic progenitor cells (median 33.4 x10^6 CD34/Kg). All the following 3 cycles of MATRix had received a rescue of stem cells (median of PBSC infusion at each cycle: 5.3 x10^6 CD34/Kg) on day + 2.
The median rise of neutrophil count (N >1000/mcl) was 13 days after second cycle and 14 days after third and fourth cycles.
Regarding toxicity the major complication were infections. 10/19 (52%) of all the infections occurred during the first cycle. According to NCCN Guidelines ® we observed 15 (79%) grade 1 and 2 infections and 4 (21%) grade 3 or 4.
Neither TRM nor ICU transferral were observed. No patient experienced cardiac or thromboembolic complication. Despite the higher frequency of complication of the first cycle no dose adjustment were made on the following cycles.
With a median of follow up of 16.1 months 50% of pts are in CR, the median OS is 13.6 months and the median PFS is 9 months.
Conclusion
With the limitation of a small series of cases, MATRix regimen with sequential infusion of PBSC and ASCT consolidation is feasible and safe.
Keyword(s): Autologous hematopoietic stem cell transplantation, CNS lymphoma, NHL