
Contributions
Abstract: PB1439
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are a rare group of non-Hodgkin lymphomas that present in skin and progress to involve extracutaneous sites in a subset of patients. The most studied subtypes, mycosis fungoides (MF) and Sézary syndrome (SS), together represent around two-thirds of all cases. Disease staging of MF/SS is multi-compartmental and utilises a modified disease specific TNMB classification. Leukemic involvement, present by definition in SS and in a proportion of MF patients, has previously been found to be an independent prognostic factor affecting overall survival, disease-specific survival and increasing the risk of disease progression (Agar 2010, Am Soc J Clin Oncol), although further research is ongoing.
As a chronic and generally incurable disease associated with disfiguring skin lesions, intractable itching, sleep disturbance, and psychosocial problems, CTCL has a serious negative effect on patient quality of life (QoL). Therefore treatment goals include reducing the burden of disease, delaying progression, and improving or preserving QoL.
Aims
This post hoc analysis of MAVORIC trial data sought to determine whether baseline blood tumour burden affected treatment effect on patient QoL, where patients classified as B0 are considered to have no blood involvement and patients classified as B1 and B2 are considered to have blood involvement.
Methods
The Phase 3 MAVORIC trial (NCT01728805) was an open-label study that compared mogamulizumab to vorinostat, with patients randomised 1:1 to each treatment arm. Mogamulizumab patients received intravenous treatment, 1.0 mg/kg weekly for the first 28-day cycle then on days 1 and 15 of subsequent cycles. Vorinostat patients received 400mg daily PO. Patient reported outcome (PRO) assessments of health-related QoL (HRQoL) were assessed using the validated Skindex-29, and FACT-G (Functional Assessment of Cancer Therapy - General) instruments. Pruritus was assessed using ItchyQoL and Pruritus Likert Scale. All PROs were administered at baseline and Skindex-29, and FACT-G were then administered at every other treatment visit (Cycle 1, 3, 5, …), and ItchyQoL and Pruritus Likert Scale were administered every 4 weeks at each cycle. Only results taken up to treatment Cycle 12 were analysed as beyond this point, patient numbers were too low to give meaningful data.
Results
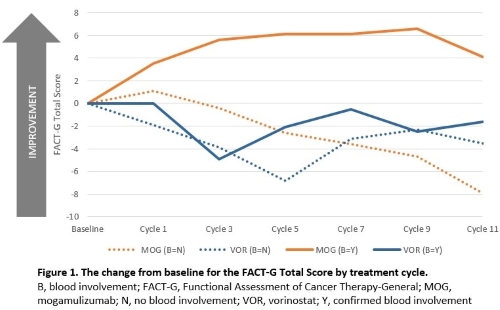
Overall, no statistically significant differences were seen between mogamulizumab and vorinostat for patients with no blood involvement. Statistically significant differences were seen for patients with blood involvement, with mogamulizumab patients seeing statistically significant improvement in Skindex-29 for treatment Cycles 3–11 in all domains (Emotional, Functional, Symptoms); and in the Functional Domain of ItchyQoL at most timepoints. In mogamulizumab-treated patients with blood involvement, the Pruritus Likert Scale Score showed a reduction in itch from Cycle 1 and a trend to reducing levels of itch over 12 cycles. The FACT-G Total Score showed greatest improvement from baseline for mogamulizumab patients with blood involvement compared to those without and all vorinostat patients (Figure 1).
Conclusion
Overall, mogamulizumab patients with blood involvement saw greater HRQoL improvement than those without blood involvement. Mogamulizumab offers QoL benefit, assessed across a number of PROs, especially to patients with blood involvement (B1 or B2).
Keyword(s): Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood, Quality of life
Abstract: PB1439
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are a rare group of non-Hodgkin lymphomas that present in skin and progress to involve extracutaneous sites in a subset of patients. The most studied subtypes, mycosis fungoides (MF) and Sézary syndrome (SS), together represent around two-thirds of all cases. Disease staging of MF/SS is multi-compartmental and utilises a modified disease specific TNMB classification. Leukemic involvement, present by definition in SS and in a proportion of MF patients, has previously been found to be an independent prognostic factor affecting overall survival, disease-specific survival and increasing the risk of disease progression (Agar 2010, Am Soc J Clin Oncol), although further research is ongoing.
As a chronic and generally incurable disease associated with disfiguring skin lesions, intractable itching, sleep disturbance, and psychosocial problems, CTCL has a serious negative effect on patient quality of life (QoL). Therefore treatment goals include reducing the burden of disease, delaying progression, and improving or preserving QoL.
Aims
This post hoc analysis of MAVORIC trial data sought to determine whether baseline blood tumour burden affected treatment effect on patient QoL, where patients classified as B0 are considered to have no blood involvement and patients classified as B1 and B2 are considered to have blood involvement.
Methods
The Phase 3 MAVORIC trial (NCT01728805) was an open-label study that compared mogamulizumab to vorinostat, with patients randomised 1:1 to each treatment arm. Mogamulizumab patients received intravenous treatment, 1.0 mg/kg weekly for the first 28-day cycle then on days 1 and 15 of subsequent cycles. Vorinostat patients received 400mg daily PO. Patient reported outcome (PRO) assessments of health-related QoL (HRQoL) were assessed using the validated Skindex-29, and FACT-G (Functional Assessment of Cancer Therapy - General) instruments. Pruritus was assessed using ItchyQoL and Pruritus Likert Scale. All PROs were administered at baseline and Skindex-29, and FACT-G were then administered at every other treatment visit (Cycle 1, 3, 5, …), and ItchyQoL and Pruritus Likert Scale were administered every 4 weeks at each cycle. Only results taken up to treatment Cycle 12 were analysed as beyond this point, patient numbers were too low to give meaningful data.
Results
Overall, no statistically significant differences were seen between mogamulizumab and vorinostat for patients with no blood involvement. Statistically significant differences were seen for patients with blood involvement, with mogamulizumab patients seeing statistically significant improvement in Skindex-29 for treatment Cycles 3–11 in all domains (Emotional, Functional, Symptoms); and in the Functional Domain of ItchyQoL at most timepoints. In mogamulizumab-treated patients with blood involvement, the Pruritus Likert Scale Score showed a reduction in itch from Cycle 1 and a trend to reducing levels of itch over 12 cycles. The FACT-G Total Score showed greatest improvement from baseline for mogamulizumab patients with blood involvement compared to those without and all vorinostat patients (Figure 1).

Conclusion
Overall, mogamulizumab patients with blood involvement saw greater HRQoL improvement than those without blood involvement. Mogamulizumab offers QoL benefit, assessed across a number of PROs, especially to patients with blood involvement (B1 or B2).
Keyword(s): Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood, Quality of life


