
Contributions
Abstract: PB1428
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are non-Hodgkin lymphomas with a variety of subtypes. Mycosis fungoides (MF) and Sézary syndrome (SS) together account for up to two-thirds of CTCL cases. Disease staging in CTCL was first comprised of tumour, lymph node and metastasis (TNM) assessments, with blood being added in 2007 due to establishment of blood tumour burden as a negative prognostic factor. Overall survival (OS) and disease-specific survival (DSS) are negatively impacted by increased blood tumour burden and risk of disease progression (RDP) is increased (Agar 2010, Am Soc J Clin Oncol). However, there is still further validation to be done for confirmation.
Aims
This post hoc analysis of MAVORIC trial data looked at the correlation between patients’ levels of blood involvement and response in the skin following treatment with either mogamulizumab or vorinostat.
Methods
MAVORIC (NCT01728805) was an open-label, phase 3 study comparing mogamulizumab and vorinostat. Patients were randomised 1:1 to each treatment arm and received either intravenous mogamulizumab 1.0 mg/kg weekly for the first 28-day cycle, then on days 1 and 15 of subsequent cycles, or oral vorinostat 400 mg once daily. Vorinostat treated patients who experienced disease toxicity or intolerable toxicity following at least one cycle could cross over to the mogamulizumab treatment arm. The Modified Severity Weighted Assessment Tool (mSWAT), was used to measure skin response in both mogamulizumab and vorinostat patients and data was stratified by blood classification. The median mSWAT percentage change per treatment cycle and best overall response by percentage change in mSWAT were examined.
Results
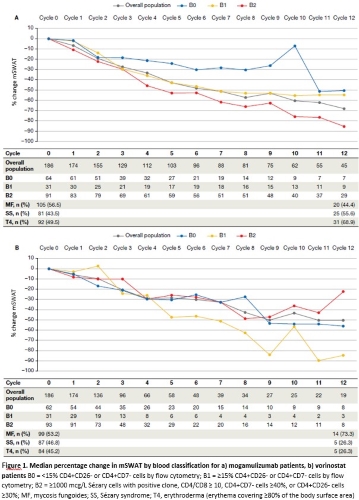
Mogamulizumab patients with B1 and B2 blood classifications experienced a greater median percentage change in mSWAT compared to patients with B0, and B2 patients experienced the greatest improvement of -86.0% by Cycle 12 (Figure 1a). The majority of patients experienced improvement in mSWAT, with the greatest degree of responses generally experienced by B1 and B2 patients.
Vorinostat patients saw an improvement in median percentage change in mSWAT, although to a lesser degree compared to mogamulizumab patients, with B2 patients showing the lowest response in skin, -22.1% by Cycle 12, compared to other blood classes (Figure 1b). Best overall mSWAT response by patient show that vorinostat patients overall experienced less improvement compared to mogamulizumab patients, with little-to-no correlation with blood class.
Conclusion
MOGA is effective in patients with blood involvement (B1 and B2), including patients with MF. Drug safety is similar between patients irrespective of level of blood involvement.
Keyword(s): Clinical data, Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood
Abstract: PB1428
Type: Publication Only
Session title: Aggressive Non-Hodgkin lymphoma - Clinical
Background
Cutaneous T-cell lymphomas (CTCL) are non-Hodgkin lymphomas with a variety of subtypes. Mycosis fungoides (MF) and Sézary syndrome (SS) together account for up to two-thirds of CTCL cases. Disease staging in CTCL was first comprised of tumour, lymph node and metastasis (TNM) assessments, with blood being added in 2007 due to establishment of blood tumour burden as a negative prognostic factor. Overall survival (OS) and disease-specific survival (DSS) are negatively impacted by increased blood tumour burden and risk of disease progression (RDP) is increased (Agar 2010, Am Soc J Clin Oncol). However, there is still further validation to be done for confirmation.
Aims
This post hoc analysis of MAVORIC trial data looked at the correlation between patients’ levels of blood involvement and response in the skin following treatment with either mogamulizumab or vorinostat.
Methods
MAVORIC (NCT01728805) was an open-label, phase 3 study comparing mogamulizumab and vorinostat. Patients were randomised 1:1 to each treatment arm and received either intravenous mogamulizumab 1.0 mg/kg weekly for the first 28-day cycle, then on days 1 and 15 of subsequent cycles, or oral vorinostat 400 mg once daily. Vorinostat treated patients who experienced disease toxicity or intolerable toxicity following at least one cycle could cross over to the mogamulizumab treatment arm. The Modified Severity Weighted Assessment Tool (mSWAT), was used to measure skin response in both mogamulizumab and vorinostat patients and data was stratified by blood classification. The median mSWAT percentage change per treatment cycle and best overall response by percentage change in mSWAT were examined.
Results
Mogamulizumab patients with B1 and B2 blood classifications experienced a greater median percentage change in mSWAT compared to patients with B0, and B2 patients experienced the greatest improvement of -86.0% by Cycle 12 (Figure 1a). The majority of patients experienced improvement in mSWAT, with the greatest degree of responses generally experienced by B1 and B2 patients.
Vorinostat patients saw an improvement in median percentage change in mSWAT, although to a lesser degree compared to mogamulizumab patients, with B2 patients showing the lowest response in skin, -22.1% by Cycle 12, compared to other blood classes (Figure 1b). Best overall mSWAT response by patient show that vorinostat patients overall experienced less improvement compared to mogamulizumab patients, with little-to-no correlation with blood class.

Conclusion
MOGA is effective in patients with blood involvement (B1 and B2), including patients with MF. Drug safety is similar between patients irrespective of level of blood involvement.
Keyword(s): Clinical data, Cutaneous T-cell lymphoma, Mycosis fungoides, Peripheral blood


