
Contributions
Abstract: PB1417
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Over the past four decades, induction chemotherapy for acute myeloid leukemia (AML) has been standardized as so-called 7+3 regimen comprising 7 days of cytarabine and 3 days of anthracycline. To improve the treatment outcomes of AML patients, several prospective trials had been conducted with regards to the dosing schedule of induction chemotherapies. The E1900 trial showed improved complete remission (CR) rate and survival with high-dose daunorubicin (90 mg/m2/d for 3 days) than standard-dose daunorubicin (45 mg/m2/d for 3 days). During the similar period, our group also conducted a randomized study comparing high-dose and standard-dose daunorubicin (90 vs. 45 mg/m2/d for 3 days) and confirmed superior CR rate and survival of high-dose daunorubicin. A randomized trial of Australian group comparing standard dose cytarabine (100 mg/m2/d for 7 days) and high-dose cytarabine (3 g/m2 q12hr for 4 days) showed improved duration of response, disease-free survival and overall survival (OS) after CR of high-dose cytarabine. Later, another randomized trial also revealed that high-dose cytarabine was associated with higher CR rate and longer survival than standard-dose cytarabine.
Aims
To optimize the induction treatment of AML, we designed a randomized study comparing high-dose cytarabine and high-dose daunorubicin for newly diagnosed AML patients.
Methods
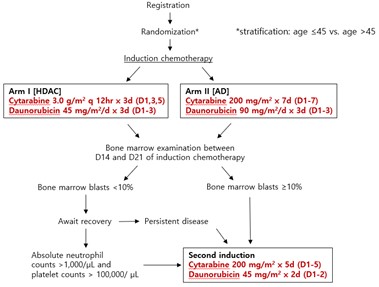
The patients with previously untreated AML who are ≤60 years old and eligible for intensive induction chemotherapy have been recruited in the study. Patients are randomized to one of the two induction treatment arms; high-dose cytarabine plus daunorubicin ([HDAC], cytarabine 3.0 g/m2 q12hr on days 1, 3, 5 plus daunorubicin 45 mg/m2/day on day 1-3) or cytarabine plus high-dose daunorubicin arm ([AD], cytarabine 200 mg/m2/day on day 1-7 plus daunorubicin 90 mg/m2/day on day 1-3). Patients who achieve CR receive consolidation chemotherapy or can proceed with hematopoietic cell transplantation (HCT) based on their genetic risk. The study was designed as a single-center, non-blind, two-arm randomized prospective controlled trial and plans to expand into a multicenter study. The primary endpoint is event-free survival, and the secondary endpoints include CR rate, cumulative incidence of relapse, regimen-related toxicities, and OS.
Results
Between March 2018 and December 2020, 103 patients were enrolled in the study; 48 in HDAC and 46 in AD arm. The median age was 49 (range, 16-60) and 61 patients were male (59.2%). Of 103 patients, 51 (49.5%) were classified into favorable genetic risk, 19 (18.4%) into intermediate risk, and 32 (31.1%) into adverse risk according to the European Leukemia Net risk stratification. FMS-like tyrosine kinase 3-internal tandem duplication and nucleophosmin mutations were detected in 18 and 29 patients, respectively. Overall, 75 and 2 patients achieved CR and CR with incomplete hematologic recovery, and 47 and 2 patients proceeded with allogeneic and autologous HCT, respectively. Eighteen patients died, and 2 died within 30 days from randomization.
Conclusion
We are currently conducting a randomized controlled trial for newly diagnosed AML patients comparing two induction chemotherapeutic regimens; high-dose cytarabine and high-dose daunorubicin. We expect to get insights from this study into optimal induction chemotherapy for AML patients and to reveal the relationship between genetic risk based on the mutational study and the benefits of each induction regimen.
Keyword(s): Acute myeloid leukemia, Induction chemotherapy
Abstract: PB1417
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Over the past four decades, induction chemotherapy for acute myeloid leukemia (AML) has been standardized as so-called 7+3 regimen comprising 7 days of cytarabine and 3 days of anthracycline. To improve the treatment outcomes of AML patients, several prospective trials had been conducted with regards to the dosing schedule of induction chemotherapies. The E1900 trial showed improved complete remission (CR) rate and survival with high-dose daunorubicin (90 mg/m2/d for 3 days) than standard-dose daunorubicin (45 mg/m2/d for 3 days). During the similar period, our group also conducted a randomized study comparing high-dose and standard-dose daunorubicin (90 vs. 45 mg/m2/d for 3 days) and confirmed superior CR rate and survival of high-dose daunorubicin. A randomized trial of Australian group comparing standard dose cytarabine (100 mg/m2/d for 7 days) and high-dose cytarabine (3 g/m2 q12hr for 4 days) showed improved duration of response, disease-free survival and overall survival (OS) after CR of high-dose cytarabine. Later, another randomized trial also revealed that high-dose cytarabine was associated with higher CR rate and longer survival than standard-dose cytarabine.
Aims
To optimize the induction treatment of AML, we designed a randomized study comparing high-dose cytarabine and high-dose daunorubicin for newly diagnosed AML patients.
Methods
The patients with previously untreated AML who are ≤60 years old and eligible for intensive induction chemotherapy have been recruited in the study. Patients are randomized to one of the two induction treatment arms; high-dose cytarabine plus daunorubicin ([HDAC], cytarabine 3.0 g/m2 q12hr on days 1, 3, 5 plus daunorubicin 45 mg/m2/day on day 1-3) or cytarabine plus high-dose daunorubicin arm ([AD], cytarabine 200 mg/m2/day on day 1-7 plus daunorubicin 90 mg/m2/day on day 1-3). Patients who achieve CR receive consolidation chemotherapy or can proceed with hematopoietic cell transplantation (HCT) based on their genetic risk. The study was designed as a single-center, non-blind, two-arm randomized prospective controlled trial and plans to expand into a multicenter study. The primary endpoint is event-free survival, and the secondary endpoints include CR rate, cumulative incidence of relapse, regimen-related toxicities, and OS.
Results
Between March 2018 and December 2020, 103 patients were enrolled in the study; 48 in HDAC and 46 in AD arm. The median age was 49 (range, 16-60) and 61 patients were male (59.2%). Of 103 patients, 51 (49.5%) were classified into favorable genetic risk, 19 (18.4%) into intermediate risk, and 32 (31.1%) into adverse risk according to the European Leukemia Net risk stratification. FMS-like tyrosine kinase 3-internal tandem duplication and nucleophosmin mutations were detected in 18 and 29 patients, respectively. Overall, 75 and 2 patients achieved CR and CR with incomplete hematologic recovery, and 47 and 2 patients proceeded with allogeneic and autologous HCT, respectively. Eighteen patients died, and 2 died within 30 days from randomization.

Conclusion
We are currently conducting a randomized controlled trial for newly diagnosed AML patients comparing two induction chemotherapeutic regimens; high-dose cytarabine and high-dose daunorubicin. We expect to get insights from this study into optimal induction chemotherapy for AML patients and to reveal the relationship between genetic risk based on the mutational study and the benefits of each induction regimen.
Keyword(s): Acute myeloid leukemia, Induction chemotherapy


