
Contributions
Abstract: PB1414
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
CCAAT/enhancer-binding protein-α (C/EBPα) is a transcription factor encoded by the CEBPA gene and involved in the control of proliferation and differentiation of myeloid progenitors. C/EBPα consists of C-terminal DNA-binding basic leucine zipper (bZIP) domain and two transactivation domains TAD 1 and TAD 2 in the NH2 terminus. Mutations in CEBPA are found in 5% to 14% of acute myeloid leukemia (AML). AML patients with biallelic mutations of CEBPA are included in “favorable” group, which determines treatment strategy. It has been shown that HP196–197 insertion (6bp) in TAD2 domain is a common inherited mutation spread in normal population; however, there has been no studies on the occurrence of this insertion in the Russian population.
Aims
To compare the frequency of 6bp insertion in TAD2 domain of CEBPA gene in AML patients with healthy and no-myeloid leukemia (CLL) control cohorts.
Methods
The study included 120 healthy controls, 117 de-novo AML patients, and 100 chronic lymphocytic leukemia (CLL) patients undergoing treatment at the National Research Center for Hematology (Moscow, Russia). Genomic DNA was isolated from bone marrow/peripheral blood samples taken at the time of diagnosis. For AML patients we also analyzed bone marrow/peripheral blood samples taken after treatment. Detection of 6bp insertion was performed on genomic DNA by PCR and fragment analysis. To amplify the region, we used a fluorescently labeled forward (F) primer FAM‐5′‐CCGGCTACCTGGACGGCAGG‐3′ and a reverse (R1) primer 5′‐CGTTGCTGTTCTTGTCCACCGACTTCTT‐3′ [T Benthaus et al, 2008]. The fragment analysis was performed on ABI3130 Genetic Analyzer (Thermofisher Scientific, USA). Comparisons between patient characteristics were performed using the Pearson χ2 test for binary variables.
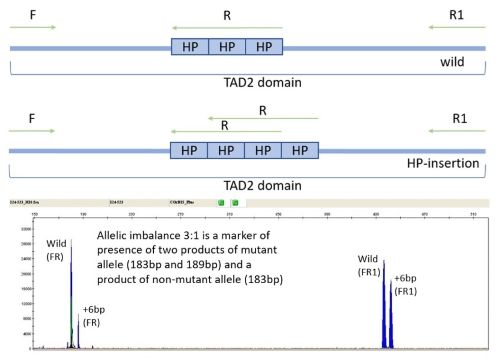
Samples with detected 6bp insertion in TAD2 domain were further analyzed to confirm that 6bp insertion was located in a region that accounts for a His–Pro duplication (HP196–197ins). Samples were amplified using fluorescently labeled forward (F) primer FAM‐5′-CCGGCTACCTGGACGGCAGG‐3′ [T Benthaus et al, 2008] and reversed (R) primer 5′-TGCGGGTGCGGGTGCGGGT‐3′ [in house design] with subsequent fragment analysis (see figure 1).
Results
6bp insertion in TAD2 domain of CEBPA gene was detected in 7 of 117 (6%) AML patients, in 9 of 120 (7,5%) healthy controls, and in 10 of 100 (10%) CLL patients; differences between these groups were not statistically significant (χ2=1.234, df=2, p=0.540). It was confirmed that 6bp insertion was HP196–197ins in all cases. In all AML patients with 6bp insertion in TAD2 domain in samples taken before treatment, this insertion was also detected after treatment as well.
Conclusion
Expected occurrence of 6bp insertion in TAD2 domain of CEBPA gene (HP196–197ins) is equal in AML patients, healthy controls, and patients with non-myeloid leukemia. Moreover, this insertion in AML patients persists after treatment, which suggests its hereditary nature. Therefore, this insertion should not be taken into account when determining the risk group and treatment strategy.
Keyword(s): Acute myeloid leukemia, CCAAT/enhancer binding protein alpha (C/EBPa), Insertion site analysis, Polymorphism
Abstract: PB1414
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
CCAAT/enhancer-binding protein-α (C/EBPα) is a transcription factor encoded by the CEBPA gene and involved in the control of proliferation and differentiation of myeloid progenitors. C/EBPα consists of C-terminal DNA-binding basic leucine zipper (bZIP) domain and two transactivation domains TAD 1 and TAD 2 in the NH2 terminus. Mutations in CEBPA are found in 5% to 14% of acute myeloid leukemia (AML). AML patients with biallelic mutations of CEBPA are included in “favorable” group, which determines treatment strategy. It has been shown that HP196–197 insertion (6bp) in TAD2 domain is a common inherited mutation spread in normal population; however, there has been no studies on the occurrence of this insertion in the Russian population.
Aims
To compare the frequency of 6bp insertion in TAD2 domain of CEBPA gene in AML patients with healthy and no-myeloid leukemia (CLL) control cohorts.
Methods
The study included 120 healthy controls, 117 de-novo AML patients, and 100 chronic lymphocytic leukemia (CLL) patients undergoing treatment at the National Research Center for Hematology (Moscow, Russia). Genomic DNA was isolated from bone marrow/peripheral blood samples taken at the time of diagnosis. For AML patients we also analyzed bone marrow/peripheral blood samples taken after treatment. Detection of 6bp insertion was performed on genomic DNA by PCR and fragment analysis. To amplify the region, we used a fluorescently labeled forward (F) primer FAM‐5′‐CCGGCTACCTGGACGGCAGG‐3′ and a reverse (R1) primer 5′‐CGTTGCTGTTCTTGTCCACCGACTTCTT‐3′ [T Benthaus et al, 2008]. The fragment analysis was performed on ABI3130 Genetic Analyzer (Thermofisher Scientific, USA). Comparisons between patient characteristics were performed using the Pearson χ2 test for binary variables.
Samples with detected 6bp insertion in TAD2 domain were further analyzed to confirm that 6bp insertion was located in a region that accounts for a His–Pro duplication (HP196–197ins). Samples were amplified using fluorescently labeled forward (F) primer FAM‐5′-CCGGCTACCTGGACGGCAGG‐3′ [T Benthaus et al, 2008] and reversed (R) primer 5′-TGCGGGTGCGGGTGCGGGT‐3′ [in house design] with subsequent fragment analysis (see figure 1).
Results
6bp insertion in TAD2 domain of CEBPA gene was detected in 7 of 117 (6%) AML patients, in 9 of 120 (7,5%) healthy controls, and in 10 of 100 (10%) CLL patients; differences between these groups were not statistically significant (χ2=1.234, df=2, p=0.540). It was confirmed that 6bp insertion was HP196–197ins in all cases. In all AML patients with 6bp insertion in TAD2 domain in samples taken before treatment, this insertion was also detected after treatment as well.

Conclusion
Expected occurrence of 6bp insertion in TAD2 domain of CEBPA gene (HP196–197ins) is equal in AML patients, healthy controls, and patients with non-myeloid leukemia. Moreover, this insertion in AML patients persists after treatment, which suggests its hereditary nature. Therefore, this insertion should not be taken into account when determining the risk group and treatment strategy.
Keyword(s): Acute myeloid leukemia, CCAAT/enhancer binding protein alpha (C/EBPa), Insertion site analysis, Polymorphism


