
Contributions
Abstract: PB1413
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
The BCL2 gene is a regulator of apoptosis, playing a key role in leukaemogenesis. BCL2 positivity in Acute Myeloid Leukaemia (AML) and its impact on prognosis is unclear. Recently, BCL2 inhibitors (Venetoclax) have shown promise in AML when used in combination with other agents. Treatment of relapsed/refractory AML is an unmet need and often has a dismal prognosis, especially in patients unsuitable for intensive chemotherapy.
Aims
Venetoclax was, until recently, made available in Ireland on a named-patient basis for use in AML. The aim of this multicentre, retrospective study was to analyse its use in this context.
Methods
Data was collected from 9 centers in Ireland. Patient and disease characteristics, prior treatment and outcomes were analysed. Real world data was also gathered regarding tolerability of Venetoclax and adverse events.
Results
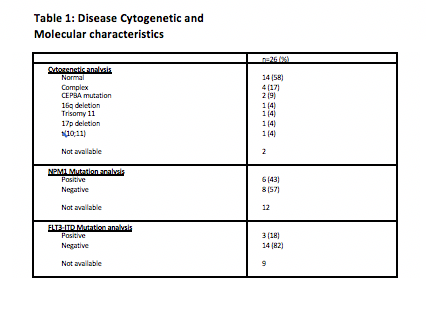
Data were collected for 26 AML patients treated with Venetoclax. Most had de novo AML (n=19; 73%). Cytogenetic and molecular analyses are outlined in Table 1. Indications for initiating Venetoclax therapy were refractory disease (n=15, 58%) and relapsed disease (35%). One patient had not tolerated Azacitadine. Venetoclax was used as a single agent in 13 patients (50%) and in combination in 11 patients (42%). Other agents used were Azacitadine (n=8, 61.5%), Decitabine (n=2, 15%), and induction chemotherapy (n=1).
When initially investigated for treatment of CLL, the main risk with initiating Venetoclax was Tumour Lysis Syndrome (TLS). This is less well characterised for patients with AML, and therefore a more rapid dose escalation regimen has been advocated. The majority (n=18, 72%) were admitted to hospital for initiation of therapy. Most patients (69%) received TLS prophylaxis with Allopurinol alone. Seven patients (27%) received Rasburicase, and one patient received intravenous fluid alone. TLS was reported in only one patient who had received Rasburicase.
Venetoclax was mostly well-tolerated. Ten patients (40%) had adverse events or side effects. Myelosuppression was the most commonly reported adverse event (n=9, 36%) and treatment was temporarily interrupted due to cytopenia in 4 of these. GCSF support was commonly used. Infections were reported in 5 patients (20%), and recurrent febrile neutropenia led to treatment discontinuation in one. Overall, seven patients required hospital admission due to Venetoclax toxicity (28%).
The median duration of treatment was 2 months (range 0.5-12). Treatment response was assessed clinically (peripheral blood counts) in 17 patients (71%) and by bone marrow aspirate and trephine biopsies in seven patients (29%). Response to treatment was variable; the majority (n=11, 46%) had progressive disease, 8 patients (33%) had stable disease, 4 patients achieved Partial Response (17%) and only one patient had a Complete Response. There was no significant difference between response rates and survival in patients treated with Venetoclax as a single agent compared to in combination (median survival from commencing Venetoclax was 2 months vs. 3 months respectively). At time of data collection, 19 patients had died (76%).
Conclusion
Venetoclax is not yet a standardised treatment for AML, and in Ireland was available only on a Compassionate Access Scheme and this has now closed. This study of real-world data indicates that Venetoclax is well-tolerated with a low risk of serious adverse events. However, OS was not as prolonged as has been reported in the literature. Based on our experience, Venetoclax may be more effective when commenced earlier in patients with relapsed/refractory disease.
Keyword(s): AML, BCL2
Abstract: PB1413
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
The BCL2 gene is a regulator of apoptosis, playing a key role in leukaemogenesis. BCL2 positivity in Acute Myeloid Leukaemia (AML) and its impact on prognosis is unclear. Recently, BCL2 inhibitors (Venetoclax) have shown promise in AML when used in combination with other agents. Treatment of relapsed/refractory AML is an unmet need and often has a dismal prognosis, especially in patients unsuitable for intensive chemotherapy.
Aims
Venetoclax was, until recently, made available in Ireland on a named-patient basis for use in AML. The aim of this multicentre, retrospective study was to analyse its use in this context.
Methods
Data was collected from 9 centers in Ireland. Patient and disease characteristics, prior treatment and outcomes were analysed. Real world data was also gathered regarding tolerability of Venetoclax and adverse events.
Results
Data were collected for 26 AML patients treated with Venetoclax. Most had de novo AML (n=19; 73%). Cytogenetic and molecular analyses are outlined in Table 1. Indications for initiating Venetoclax therapy were refractory disease (n=15, 58%) and relapsed disease (35%). One patient had not tolerated Azacitadine. Venetoclax was used as a single agent in 13 patients (50%) and in combination in 11 patients (42%). Other agents used were Azacitadine (n=8, 61.5%), Decitabine (n=2, 15%), and induction chemotherapy (n=1).
When initially investigated for treatment of CLL, the main risk with initiating Venetoclax was Tumour Lysis Syndrome (TLS). This is less well characterised for patients with AML, and therefore a more rapid dose escalation regimen has been advocated. The majority (n=18, 72%) were admitted to hospital for initiation of therapy. Most patients (69%) received TLS prophylaxis with Allopurinol alone. Seven patients (27%) received Rasburicase, and one patient received intravenous fluid alone. TLS was reported in only one patient who had received Rasburicase.
Venetoclax was mostly well-tolerated. Ten patients (40%) had adverse events or side effects. Myelosuppression was the most commonly reported adverse event (n=9, 36%) and treatment was temporarily interrupted due to cytopenia in 4 of these. GCSF support was commonly used. Infections were reported in 5 patients (20%), and recurrent febrile neutropenia led to treatment discontinuation in one. Overall, seven patients required hospital admission due to Venetoclax toxicity (28%).
The median duration of treatment was 2 months (range 0.5-12). Treatment response was assessed clinically (peripheral blood counts) in 17 patients (71%) and by bone marrow aspirate and trephine biopsies in seven patients (29%). Response to treatment was variable; the majority (n=11, 46%) had progressive disease, 8 patients (33%) had stable disease, 4 patients achieved Partial Response (17%) and only one patient had a Complete Response. There was no significant difference between response rates and survival in patients treated with Venetoclax as a single agent compared to in combination (median survival from commencing Venetoclax was 2 months vs. 3 months respectively). At time of data collection, 19 patients had died (76%).

Conclusion
Venetoclax is not yet a standardised treatment for AML, and in Ireland was available only on a Compassionate Access Scheme and this has now closed. This study of real-world data indicates that Venetoclax is well-tolerated with a low risk of serious adverse events. However, OS was not as prolonged as has been reported in the literature. Based on our experience, Venetoclax may be more effective when commenced earlier in patients with relapsed/refractory disease.
Keyword(s): AML, BCL2


