
Contributions
Abstract: PB1406
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Standard treatment with chemotherapy of patients with acute myeloid leukemia (AML) can induce remission; however, despite previous evaluation in clinical trials, maintenance/post-remission therapy is currently not a standard approach in the treatment of AML. Variation in therapy approval, reimbursement, and clinical practice may influence regional differences across Europe. Currently, recommendations from European organizations on maintenance therapy lack consistency, and the use of maintenance in the real-world setting in Europe is poorly understood.
Aims
Identify European guideline recommendations and real-world evidence on the use of maintenance/post-remission therapy for adult patients with AML who had achieved complete remission after intensive chemotherapy induction.
Methods
Two targeted literature reviews were conducted by an information specialist via database searches of MEDLINE® and EMBASE using controlled vocabulary (eg, “leukemia, myeloid, acute”) and keywords (eg, “observational study” and “retrospective”); search results were not limited by language, region, or publication year. Additionally, literature searches of region-specific hematology conferences were conducted to identify unpublished treatment guidelines and real-world studies. Webpages of European health ministries were also searched for treatment guidelines. Identified records were reviewed at the title/abstract and full-text levels, with relevant information from included studies extracted.
Results
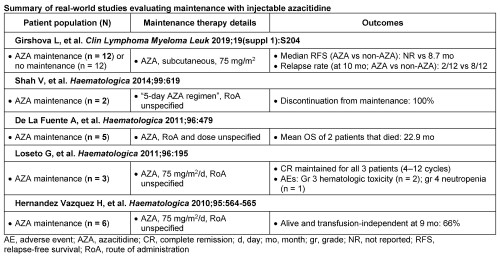
In total, for treatment guidelines, 116 records were identified from database searches and 13 from supplementary searches. For real-world studies, 482 records were identified from database searches. After screening, 14 records were included in the review of guidelines (3 pan-European, 11 region-specific) and 5 studies were included in the review of real-world studies (all limited to conference abstracts in 2010–2019, with no full publications identified; locations: 2 in Spain, 1 each in Russia, the UK, and Italy). Guidelines regarding induction and consolidation with chemotherapy were consistent, but recommendations for maintenance therapy varied (recommended [n = 7], not recommended [n = 5], and no guidance [n = 2]). In the guidelines that recommended maintenance therapy, the most common therapy was midostaurin (n = 4). Other therapies included azacitidine (n = 1) and histamine dihydrochloride (n = 1); notably, 2 guidelines recommended use of post-remission maintenance therapy, but the therapy was not specified. For the real-world studies, all 5 studies evaluated the efficacy of maintenance with injectable azacitidine (Table). Overall, the design of studies was heterogenous with limited sample size (range: n = 3–24), with only one study providing a direct comparison of treatment effects between maintenance options (injectable azacitidine maintenance vs no maintenance). Reported outcomes varied across studies, with survival at study follow-up and relapse rates being the most frequently reported. In the largest study (n = 24), the relapse rate (at 10 months) with azacitidine maintenance was 17% (n = 2/12) vs 67% (n = 8/12) with no maintenance.
Conclusion
There is a lack of consistency in recommendations for maintenance therapy across European guidelines, and very limited evidence exists in the published literature on the use of maintenance after first complete remission in the real-world setting, thereby highlighting an unmet need in the treatment of AML.
Keyword(s): Acute myeloid leukemia, Azacitidine, Maintenance, Systematic review
Abstract: PB1406
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Standard treatment with chemotherapy of patients with acute myeloid leukemia (AML) can induce remission; however, despite previous evaluation in clinical trials, maintenance/post-remission therapy is currently not a standard approach in the treatment of AML. Variation in therapy approval, reimbursement, and clinical practice may influence regional differences across Europe. Currently, recommendations from European organizations on maintenance therapy lack consistency, and the use of maintenance in the real-world setting in Europe is poorly understood.
Aims
Identify European guideline recommendations and real-world evidence on the use of maintenance/post-remission therapy for adult patients with AML who had achieved complete remission after intensive chemotherapy induction.
Methods
Two targeted literature reviews were conducted by an information specialist via database searches of MEDLINE® and EMBASE using controlled vocabulary (eg, “leukemia, myeloid, acute”) and keywords (eg, “observational study” and “retrospective”); search results were not limited by language, region, or publication year. Additionally, literature searches of region-specific hematology conferences were conducted to identify unpublished treatment guidelines and real-world studies. Webpages of European health ministries were also searched for treatment guidelines. Identified records were reviewed at the title/abstract and full-text levels, with relevant information from included studies extracted.
Results
In total, for treatment guidelines, 116 records were identified from database searches and 13 from supplementary searches. For real-world studies, 482 records were identified from database searches. After screening, 14 records were included in the review of guidelines (3 pan-European, 11 region-specific) and 5 studies were included in the review of real-world studies (all limited to conference abstracts in 2010–2019, with no full publications identified; locations: 2 in Spain, 1 each in Russia, the UK, and Italy). Guidelines regarding induction and consolidation with chemotherapy were consistent, but recommendations for maintenance therapy varied (recommended [n = 7], not recommended [n = 5], and no guidance [n = 2]). In the guidelines that recommended maintenance therapy, the most common therapy was midostaurin (n = 4). Other therapies included azacitidine (n = 1) and histamine dihydrochloride (n = 1); notably, 2 guidelines recommended use of post-remission maintenance therapy, but the therapy was not specified. For the real-world studies, all 5 studies evaluated the efficacy of maintenance with injectable azacitidine (Table). Overall, the design of studies was heterogenous with limited sample size (range: n = 3–24), with only one study providing a direct comparison of treatment effects between maintenance options (injectable azacitidine maintenance vs no maintenance). Reported outcomes varied across studies, with survival at study follow-up and relapse rates being the most frequently reported. In the largest study (n = 24), the relapse rate (at 10 months) with azacitidine maintenance was 17% (n = 2/12) vs 67% (n = 8/12) with no maintenance.

Conclusion
There is a lack of consistency in recommendations for maintenance therapy across European guidelines, and very limited evidence exists in the published literature on the use of maintenance after first complete remission in the real-world setting, thereby highlighting an unmet need in the treatment of AML.
Keyword(s): Acute myeloid leukemia, Azacitidine, Maintenance, Systematic review


