
Contributions
Abstract: PB1402
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
ALFA group and some others have shown gemtuzumab (GO) to be effective in low risk acute myeloid leukemia (AML). However, limited data on GO efficacy are available in relapse/refractory (R/R) setting outside clinical trials.
Aims
To assess the efficacy and safety of GO in combination with chemotherapy 'FLAG+/-Ida' and azacitidine in patients with R/R AML in real clinical practice.
Methods
The study included 32 patients (16 male, 16 female patients, median age–44 years (23-83 y.o.)). 46,8% (15/32) with refractory and 53,2% (17/32) with relapsed AML. 46,8% (15/32) patients received “GO-FLAG+/-Ida”, 53,2% patients (17/32) received “GO-azacitidine”. “GO-azacitidine” was given to patients unfit for intensive chemotherapy (advanced age, low somatic status, relapse or refractoriness to high-dose chemotherapy). 19 (59,3%) patients underwent the BMT subsequently.
Results
ORR (CR, CRMRD-; CRi, MFLS) was 59,4% (19/32). Early mortality was 9,4 % (3/32). OR rate was not associated with expression of CD33 (p=0,452), gender (р=0,6), age (р=0,145), prognostic risk group (р=0,646), number of previous lines (р=0,482), number of relapses (р=0,489). 80% patients, refractory to FLAG, achieved a response after “GO-azacitidine” with subsequent BMT. Non-eligible for high-dose chemotherapy patients obtained “GO-azacitidine” regimen. 66,6% of them improved their somatic status and subsequently undergone BMT. 1 of 2 patients with a relapse after high-dose chemotherapy achieved a response, BMT was performed in 2 patients. The rate of toxicity was not significantly different between the groups. No cases of VOD were reported. The rate of infusion-related complications was 46,7% (7/15) (40%>grade 1-2, 6,7%>grade 3) in the “GO-FLAG+/-Ida” group and 35,3% patients (6/17) (29,4%>grade 1-2, 5,9%>grade 4) in the “GO-azacitidine” group. SAEs were reported in 33,3% patients in the “GO- FLAG+/-Ida” group, with 100% having sepsis, and 35,3% in the “GO-azacitidine” group (66,6%>sepsis, 16,7%>acute cardiovascular failure, 16,7%>acute respiratory). No significant difference was observed in grade 3-4 neutropenia between the groups. The duration of thrombocytopenia was higher in patients who received GO in combination with “FLAG+/-Ida”, however, no significant differences were observed. The median of OS was 31,4 months, the median of RFS was 13,3 months. The median of OS was 31,4 months, the median of RFS was 13,3 months. OS was higher in the group of patients responded to therapy than in non-responder to therapy (median not achieved versus 17,97 months, respectively, p=0,044) (Fig. 1).
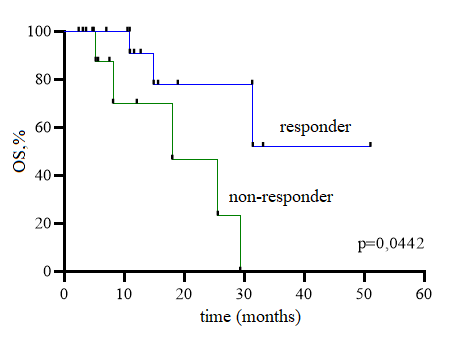
Conclusion
The combinations GO with FLAG/FLAG-Ida and azacitidine have shown to be effective regimen in patients with relapsed and refractory AML. The remission rate did not depend on the risk group, gender, age, CD33 expression, number of previous lines of therapy, number of relapses. “GO-azacitidine” demonstrated efficacy and tolerability in patients, refractory to high dose chemotherapy and in patients with poor performance status, enabling some patients to proceed to the BMT. The rate of toxicity, the duration of blood levels recovery was not statistically different between the groups, however, there was a higher duration of thrombocytopenia in “GO-FLAG+/-Ida” group. There was a higher OS in the GO responders in contrast to non-responders.
Keyword(s): AML, Gemtuzumab ozogamicin, Refractory, Relapse
Abstract: PB1402
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
ALFA group and some others have shown gemtuzumab (GO) to be effective in low risk acute myeloid leukemia (AML). However, limited data on GO efficacy are available in relapse/refractory (R/R) setting outside clinical trials.
Aims
To assess the efficacy and safety of GO in combination with chemotherapy 'FLAG+/-Ida' and azacitidine in patients with R/R AML in real clinical practice.
Methods
The study included 32 patients (16 male, 16 female patients, median age–44 years (23-83 y.o.)). 46,8% (15/32) with refractory and 53,2% (17/32) with relapsed AML. 46,8% (15/32) patients received “GO-FLAG+/-Ida”, 53,2% patients (17/32) received “GO-azacitidine”. “GO-azacitidine” was given to patients unfit for intensive chemotherapy (advanced age, low somatic status, relapse or refractoriness to high-dose chemotherapy). 19 (59,3%) patients underwent the BMT subsequently.
Results
ORR (CR, CRMRD-; CRi, MFLS) was 59,4% (19/32). Early mortality was 9,4 % (3/32). OR rate was not associated with expression of CD33 (p=0,452), gender (р=0,6), age (р=0,145), prognostic risk group (р=0,646), number of previous lines (р=0,482), number of relapses (р=0,489). 80% patients, refractory to FLAG, achieved a response after “GO-azacitidine” with subsequent BMT. Non-eligible for high-dose chemotherapy patients obtained “GO-azacitidine” regimen. 66,6% of them improved their somatic status and subsequently undergone BMT. 1 of 2 patients with a relapse after high-dose chemotherapy achieved a response, BMT was performed in 2 patients. The rate of toxicity was not significantly different between the groups. No cases of VOD were reported. The rate of infusion-related complications was 46,7% (7/15) (40%>grade 1-2, 6,7%>grade 3) in the “GO-FLAG+/-Ida” group and 35,3% patients (6/17) (29,4%>grade 1-2, 5,9%>grade 4) in the “GO-azacitidine” group. SAEs were reported in 33,3% patients in the “GO- FLAG+/-Ida” group, with 100% having sepsis, and 35,3% in the “GO-azacitidine” group (66,6%>sepsis, 16,7%>acute cardiovascular failure, 16,7%>acute respiratory). No significant difference was observed in grade 3-4 neutropenia between the groups. The duration of thrombocytopenia was higher in patients who received GO in combination with “FLAG+/-Ida”, however, no significant differences were observed. The median of OS was 31,4 months, the median of RFS was 13,3 months. The median of OS was 31,4 months, the median of RFS was 13,3 months. OS was higher in the group of patients responded to therapy than in non-responder to therapy (median not achieved versus 17,97 months, respectively, p=0,044) (Fig. 1).

Conclusion
The combinations GO with FLAG/FLAG-Ida and azacitidine have shown to be effective regimen in patients with relapsed and refractory AML. The remission rate did not depend on the risk group, gender, age, CD33 expression, number of previous lines of therapy, number of relapses. “GO-azacitidine” demonstrated efficacy and tolerability in patients, refractory to high dose chemotherapy and in patients with poor performance status, enabling some patients to proceed to the BMT. The rate of toxicity, the duration of blood levels recovery was not statistically different between the groups, however, there was a higher duration of thrombocytopenia in “GO-FLAG+/-Ida” group. There was a higher OS in the GO responders in contrast to non-responders.
Keyword(s): AML, Gemtuzumab ozogamicin, Refractory, Relapse


