
Contributions
Abstract: PB1399
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Acute promyelocytic leukemia with PML-RARa (APL) is a subgroup of acute myeloid leukemia with a favorable prognosis after the advent of differentiation agents. The present standard of care for non-high risk APL is the chemo-free combination therapy of trans retinoic acid (ATRA) plus arsenic trioxide (ATO). These therapies comprise a specific toxicity profile.
Aims
Description of one center therapy experience. Comparison of outcomes and toxicities between two different treatment regimens.
Methods
A retrospective single-center study including all patients (pts) diagnosed with de novo APL between June/2010 and June/2020. Low and intermediate risk groups (low/int), according to the Sanz Risk Score, were analyzed together. Prior to 2017, the PETHEMA APL 2005 protocol (and 2012 update) was in use to all patients; from 2017 onwards, pts were progressively switched to the AML17 protocol. Early and late death refer to events occurring during the 30 days after diagnosis or later, respectively.
Results
We identified 32 pts with a median age at diagnosis of 46y (range 22-80y) and female predominance (n=20, 62.5%). The majority presented with bleeding (n=26, 81.3%) and had low/int risk criteria (n= 26, 81.2%).
High-risk group: 5 pts completed induction with ATRA+Idarrubicin (AIDA), 1 pt with ATRA in monotherapy. Low/int-risk group: until 2017, 11 pts (42.3%) completed induction with AIDA; after 2017, 10 pts (38.5%) were treated with ATRA+ATO; the remaining 5 pts (received only ATRA or ATO in monotherapy (and 1 pt wasn’t treated) due to early complications.
All pts evaluated at the end of the induction cycle (n=27) met complete hematologic response criteria (CHR). Pts received consolidation and maintenance therapy according to the established protocols with the exception of 7 pts due to complications during treatment (n=5 AIDA, n=2 ATRA+ATO).
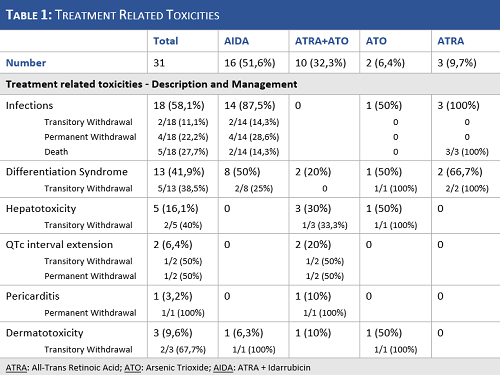
The main toxicities observed in our sample and their management are reported in Table 1.
With a median follow-up time of 35 months (52.4 and 19.8 months in AIDA and ATRA+ATO, respectively), the median overall survival was 32 months (51.5 and 22 months in AIDA and ATRA+ATO, respectively) and the overall mortality rate was 25% (n=8). Four of the above pts were undertreated (3 early deaths and 1 late death) and the remaining 4 pts were treated according to the AIDA protocol (1 early death and 3 late deaths; n=3 int/low and n=1 high risk). Five pts relapsed (4 in the AIDA group and 1 in the ATRA+ATO), with a median time to relapse of 13 months. Four of these pts were rescued (n=2 ATRA+ATO; n=1 ATO monotherapy; n=1 AIDA) and consolidated with autologous stem cell transplantation and remain in molecular response.
Conclusion
We present the real-life experience of one small center in the management of APL across a long and changing period. We underline the inclusion of undertreated pts due to comorbidities and early life-threatening complications. We confirmed the differences in toxicity profiles of chemotherapy-based and -free regimens, with infections in almost all pts in the AIDA group and hepatotoxicity and cardiac complications in a substantial part of the ATRA+ATO treated pts. Both regimens induced CHR in all pts post-induction, but we observed a trend for higher relapse risk in the AIDA group, at least partially explained by the short follow-up and low number of pts in the ATRA+ATO group.
Keyword(s): Acute promyelocytic leukemia, ALL-trans retinoic acid (ATRA), Arsenic trioxide, Toxicity
Abstract: PB1399
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Acute promyelocytic leukemia with PML-RARa (APL) is a subgroup of acute myeloid leukemia with a favorable prognosis after the advent of differentiation agents. The present standard of care for non-high risk APL is the chemo-free combination therapy of trans retinoic acid (ATRA) plus arsenic trioxide (ATO). These therapies comprise a specific toxicity profile.
Aims
Description of one center therapy experience. Comparison of outcomes and toxicities between two different treatment regimens.
Methods
A retrospective single-center study including all patients (pts) diagnosed with de novo APL between June/2010 and June/2020. Low and intermediate risk groups (low/int), according to the Sanz Risk Score, were analyzed together. Prior to 2017, the PETHEMA APL 2005 protocol (and 2012 update) was in use to all patients; from 2017 onwards, pts were progressively switched to the AML17 protocol. Early and late death refer to events occurring during the 30 days after diagnosis or later, respectively.
Results
We identified 32 pts with a median age at diagnosis of 46y (range 22-80y) and female predominance (n=20, 62.5%). The majority presented with bleeding (n=26, 81.3%) and had low/int risk criteria (n= 26, 81.2%).
High-risk group: 5 pts completed induction with ATRA+Idarrubicin (AIDA), 1 pt with ATRA in monotherapy. Low/int-risk group: until 2017, 11 pts (42.3%) completed induction with AIDA; after 2017, 10 pts (38.5%) were treated with ATRA+ATO; the remaining 5 pts (received only ATRA or ATO in monotherapy (and 1 pt wasn’t treated) due to early complications.
All pts evaluated at the end of the induction cycle (n=27) met complete hematologic response criteria (CHR). Pts received consolidation and maintenance therapy according to the established protocols with the exception of 7 pts due to complications during treatment (n=5 AIDA, n=2 ATRA+ATO).
The main toxicities observed in our sample and their management are reported in Table 1.
With a median follow-up time of 35 months (52.4 and 19.8 months in AIDA and ATRA+ATO, respectively), the median overall survival was 32 months (51.5 and 22 months in AIDA and ATRA+ATO, respectively) and the overall mortality rate was 25% (n=8). Four of the above pts were undertreated (3 early deaths and 1 late death) and the remaining 4 pts were treated according to the AIDA protocol (1 early death and 3 late deaths; n=3 int/low and n=1 high risk). Five pts relapsed (4 in the AIDA group and 1 in the ATRA+ATO), with a median time to relapse of 13 months. Four of these pts were rescued (n=2 ATRA+ATO; n=1 ATO monotherapy; n=1 AIDA) and consolidated with autologous stem cell transplantation and remain in molecular response.

Conclusion
We present the real-life experience of one small center in the management of APL across a long and changing period. We underline the inclusion of undertreated pts due to comorbidities and early life-threatening complications. We confirmed the differences in toxicity profiles of chemotherapy-based and -free regimens, with infections in almost all pts in the AIDA group and hepatotoxicity and cardiac complications in a substantial part of the ATRA+ATO treated pts. Both regimens induced CHR in all pts post-induction, but we observed a trend for higher relapse risk in the AIDA group, at least partially explained by the short follow-up and low number of pts in the ATRA+ATO group.
Keyword(s): Acute promyelocytic leukemia, ALL-trans retinoic acid (ATRA), Arsenic trioxide, Toxicity


