Contributions
Abstract: PB1394
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Recent genomic studies showed that IDH1/2 are frequently mutated in AML at about 20% and pre-clinical studies demonstrated that aberrant 2-HG elevation driven by the mutant IDH1/2 proteins plays a pivotal role in AML development by suppressing 5mC-to-5hmC conversion. Subsequent clinical trials of IDH1/2 inhibitors demonstrated promising outcomes in IDH1/2mut AML patients.
Aims
In this single institutional retrospective study, we explored the efficacy and safety outcomes of IDH1/2mut AML patients treated with Ivosidenib or Enasidenib.
Methods
We retrospectively identified AML patients who had IDH1/2 somatic mutations based on NGS assessments. Clinical and demographic data were extracted from the medical records. Statistical analyses were performed using GraphPad Prism (v.7.03) and SPSS (v.24.0).
Results
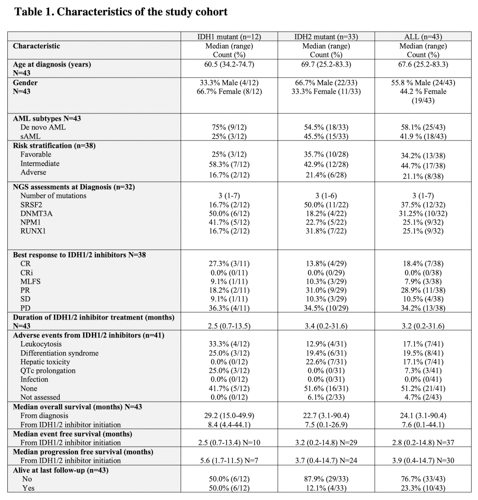
A total of 43 (IDH1mut, n=12; IDH2mut, n=33; both IDH1/2mut, n=2) patients were included in the study. Median age at AML diagnosis was 67.6 (24.2-83.3) years and 24 (55.8%) patients were male. Eighteen (42%) patients had secondary AML and 13 (34.2%), 17 (44.7%), and 8 (21.1%) patients had favorable, intermediate, and adverse risk, respectively. A total of 23 (53.5%) and 9 (20.9%) patients received intensive chemotherapy and hypomethylating agents as their 1st line therapy. One patient received Enasidenib as the 1st line therapy and the rest of the patients had relapsed/refractory disease prior to IDH1/2 inhibitor therapy. Median number of treatment prior to IDH1/2 inhibitors was 4 (0-8). The median duration of IDH1/2 inhibitor treatment was 3.2 (0.2-31.6) months (IDH1mut, 2.5 [0.7-13.5]; IDH2mut, 3.4 [0.2-31.6]). Treatment response was assessed in 38 patients and 18 had overall response (CR, n=7 [18.4%]; PR, n=11 [28.9%]). Of note, among 33 (76.7%) patients who had NGS assessments prior to IDH1/2 inhibitor initiation, a total of 13 patients had concurrent somatic mutations in the survival pathway that include FLT3, KRAS, NRAS, and PTPN11. The overall response rate in these patients was not statistically different compared to patients who did not have survival pathway mutations (38.5% vs. 40%, p>0.05). The median PFS was 3.9 (0.4-14.7) months (IDH1mut, 5.6 [1.7-11.5] vs. IDH2mut, 3.7 [0.4-14.7], p>0.05) and median OS was 7.6 (0.4-44.1) months. The most common reason for IDH1/2 inhibitor discontinuation was disease progression (n=21) followed by adverse events (n=3) and allogeneic transplant (n=2). The adverse events were assessed in 41 patients and the most common adverse events were differentiation syndrome (IDH1mut, n=3; IDH2mut, n=5) and leukocytosis (IDH1mut, n=4; IDH2mut, n=4) followed by hepatic toxicity (IDH2mut n=7), and QTc prolongation (IDH1mut, n=3).
Conclusion
Our study indicates that IDH1/2 inhibitors remain a viable option for the refractory/relapsed IDH1/2mut AML. However, significant number of patients failed to show any response and many of the patients who showed initial response had short response duration. These findings warrant further studies to identify underlying resistance mechanisms of IDH1/2 inhibitors and the optimal combination therapeutic strategies.
Keyword(s): Acute myeloid leukemia, Enasidenib, Ivosidenib, Somatic mutation
Abstract: PB1394
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Recent genomic studies showed that IDH1/2 are frequently mutated in AML at about 20% and pre-clinical studies demonstrated that aberrant 2-HG elevation driven by the mutant IDH1/2 proteins plays a pivotal role in AML development by suppressing 5mC-to-5hmC conversion. Subsequent clinical trials of IDH1/2 inhibitors demonstrated promising outcomes in IDH1/2mut AML patients.
Aims
In this single institutional retrospective study, we explored the efficacy and safety outcomes of IDH1/2mut AML patients treated with Ivosidenib or Enasidenib.
Methods
We retrospectively identified AML patients who had IDH1/2 somatic mutations based on NGS assessments. Clinical and demographic data were extracted from the medical records. Statistical analyses were performed using GraphPad Prism (v.7.03) and SPSS (v.24.0).
Results
A total of 43 (IDH1mut, n=12; IDH2mut, n=33; both IDH1/2mut, n=2) patients were included in the study. Median age at AML diagnosis was 67.6 (24.2-83.3) years and 24 (55.8%) patients were male. Eighteen (42%) patients had secondary AML and 13 (34.2%), 17 (44.7%), and 8 (21.1%) patients had favorable, intermediate, and adverse risk, respectively. A total of 23 (53.5%) and 9 (20.9%) patients received intensive chemotherapy and hypomethylating agents as their 1st line therapy. One patient received Enasidenib as the 1st line therapy and the rest of the patients had relapsed/refractory disease prior to IDH1/2 inhibitor therapy. Median number of treatment prior to IDH1/2 inhibitors was 4 (0-8). The median duration of IDH1/2 inhibitor treatment was 3.2 (0.2-31.6) months (IDH1mut, 2.5 [0.7-13.5]; IDH2mut, 3.4 [0.2-31.6]). Treatment response was assessed in 38 patients and 18 had overall response (CR, n=7 [18.4%]; PR, n=11 [28.9%]). Of note, among 33 (76.7%) patients who had NGS assessments prior to IDH1/2 inhibitor initiation, a total of 13 patients had concurrent somatic mutations in the survival pathway that include FLT3, KRAS, NRAS, and PTPN11. The overall response rate in these patients was not statistically different compared to patients who did not have survival pathway mutations (38.5% vs. 40%, p>0.05). The median PFS was 3.9 (0.4-14.7) months (IDH1mut, 5.6 [1.7-11.5] vs. IDH2mut, 3.7 [0.4-14.7], p>0.05) and median OS was 7.6 (0.4-44.1) months. The most common reason for IDH1/2 inhibitor discontinuation was disease progression (n=21) followed by adverse events (n=3) and allogeneic transplant (n=2). The adverse events were assessed in 41 patients and the most common adverse events were differentiation syndrome (IDH1mut, n=3; IDH2mut, n=5) and leukocytosis (IDH1mut, n=4; IDH2mut, n=4) followed by hepatic toxicity (IDH2mut n=7), and QTc prolongation (IDH1mut, n=3).
Conclusion
Our study indicates that IDH1/2 inhibitors remain a viable option for the refractory/relapsed IDH1/2mut AML. However, significant number of patients failed to show any response and many of the patients who showed initial response had short response duration. These findings warrant further studies to identify underlying resistance mechanisms of IDH1/2 inhibitors and the optimal combination therapeutic strategies.
Keyword(s): Acute myeloid leukemia, Enasidenib, Ivosidenib, Somatic mutation