Contributions
Abstract: PB1390
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Few studies have assessed treatment patterns and healthcare resource utilization (HRU) in patients with acute myeloid leukemia (AML) by FMS-like tyrosine kinase 3 (FLT3) mutation status (ie, FLT3 gene mutation [FLT3mut+] vs FLT3 wild-type [FLT3wt]), the most frequently identified molecular mutation in AML. The rapidly evolving treatment landscape and high cost of care in AML widen the persistent gap in detailed real-world evidence for this population.
Aims
To describe and compare baseline characteristics, real-world treatment patterns, and AML-related HRU by FLT3 mutation status in patients newly diagnosed with AML.
Methods
Retrospective chart-level data were abstracted by hematology/oncology physicians that participate in the IQVIA Oncology Panel Survey in Canada, the UK, France, Germany, Italy, and Spain. Patients newly diagnosed with FLT3mut+ or FLT3wt AML from 01/09/2017-30/06/2019 were selected for inclusion (study period: 01/01/2017-31/03/2020). The index date was defined as the date of first treatment after initial AML diagnosis; follow-up ended at death, end of available data, or end of study. Treatment patterns were assessed from the index date until the end of follow-up; HRU was assessed from the diagnosis date through the end of follow-up.
Results
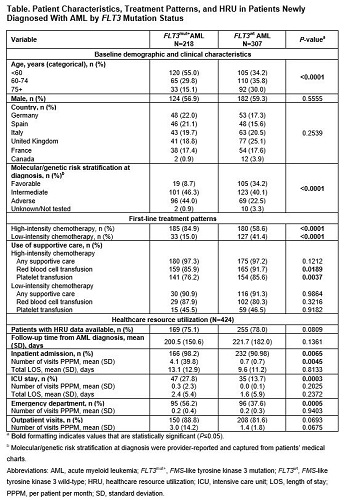
Data from 525 patients were included (FLT3mut+, 41.5%; FLT3wt, 58.5%; Table). In both cohorts, >80% of patients started AML treatment in 2019 and had a median follow-up of 4.4 months. Patients with FLT3mut+ vs FLT3wt AML were significantly younger and more likely to be in the adverse molecular/genetic risk group at diagnosis (both P<0.0001). Most patients received only one line of therapy during the follow-up period (FLT3mut+, 89.4%; FLT3wt, 90.6%). A higher proportion of patients with FLT3mut+ (84.9%, n=185) than FLT3wt AML (58.6%, n=180) received first-line high-intensity chemotherapy (HIC). The predominant HIC regimen was 7+3 (7 days cytarabine + 3 days anthracycline) (FLT3mut+, 89.2%; FLT3wt, 81.1%)—most commonly 7+3 with FLT3 inhibitor (predominantly midostaurin) (43.8%) in FLT3mut+ patients and 7+3 alone (72.0%) in FLT3wt patients. Nearly all patients who received low-intensity chemotherapy (LIC) had FLT3wt AML; the predominant LIC regimen was a hypomethylating agent with or without venetoclax (FLT3mut+, 51.5%; FLT3wt, 67.7%). Most patients received supportive care during treatment, and transfusion of blood products was common (Table). HRU was assessed in 424 patients (80.8%). Patients with FLT3mut+ vs FLT3wt AML had ≥4 times more inpatient admissions (P=0.0045) and higher proportions of intensive care unit admissions (P=0.0003) and emergency department visits (P=0.0005).
Conclusion
These real-world data illustrate the differences in demographic and clinical characteristics, first-line treatment patterns, and HRU between FLT3mut+ and FLT3wt AML patient populations in Canada, the UK, France, Germany, Italy, and Spain. Age and FLT3 mutation status appeared to influence the type of induction regimen used; although most patients received HIC, FLT3mut+ vs FLT3wt patients were significantly younger and a higher percentage received HIC. Nearly half of FLT3mut+ patients receiving HIC also received a FLT3 inhibitor. The high use of supportive care and inpatient and outpatient HRU highlight the significant morbidity and economic burden associated with managing AML, the importance of knowing patients’ FLT3 mutation status, and the need to further understand how patients with FLT3mut+ AML may differ from other patients with AML.
Keyword(s): Mutation status
Abstract: PB1390
Type: Publication Only
Session title: Acute myeloid leukemia - Clinical
Background
Few studies have assessed treatment patterns and healthcare resource utilization (HRU) in patients with acute myeloid leukemia (AML) by FMS-like tyrosine kinase 3 (FLT3) mutation status (ie, FLT3 gene mutation [FLT3mut+] vs FLT3 wild-type [FLT3wt]), the most frequently identified molecular mutation in AML. The rapidly evolving treatment landscape and high cost of care in AML widen the persistent gap in detailed real-world evidence for this population.
Aims
To describe and compare baseline characteristics, real-world treatment patterns, and AML-related HRU by FLT3 mutation status in patients newly diagnosed with AML.
Methods
Retrospective chart-level data were abstracted by hematology/oncology physicians that participate in the IQVIA Oncology Panel Survey in Canada, the UK, France, Germany, Italy, and Spain. Patients newly diagnosed with FLT3mut+ or FLT3wt AML from 01/09/2017-30/06/2019 were selected for inclusion (study period: 01/01/2017-31/03/2020). The index date was defined as the date of first treatment after initial AML diagnosis; follow-up ended at death, end of available data, or end of study. Treatment patterns were assessed from the index date until the end of follow-up; HRU was assessed from the diagnosis date through the end of follow-up.
Results
Data from 525 patients were included (FLT3mut+, 41.5%; FLT3wt, 58.5%; Table). In both cohorts, >80% of patients started AML treatment in 2019 and had a median follow-up of 4.4 months. Patients with FLT3mut+ vs FLT3wt AML were significantly younger and more likely to be in the adverse molecular/genetic risk group at diagnosis (both P<0.0001). Most patients received only one line of therapy during the follow-up period (FLT3mut+, 89.4%; FLT3wt, 90.6%). A higher proportion of patients with FLT3mut+ (84.9%, n=185) than FLT3wt AML (58.6%, n=180) received first-line high-intensity chemotherapy (HIC). The predominant HIC regimen was 7+3 (7 days cytarabine + 3 days anthracycline) (FLT3mut+, 89.2%; FLT3wt, 81.1%)—most commonly 7+3 with FLT3 inhibitor (predominantly midostaurin) (43.8%) in FLT3mut+ patients and 7+3 alone (72.0%) in FLT3wt patients. Nearly all patients who received low-intensity chemotherapy (LIC) had FLT3wt AML; the predominant LIC regimen was a hypomethylating agent with or without venetoclax (FLT3mut+, 51.5%; FLT3wt, 67.7%). Most patients received supportive care during treatment, and transfusion of blood products was common (Table). HRU was assessed in 424 patients (80.8%). Patients with FLT3mut+ vs FLT3wt AML had ≥4 times more inpatient admissions (P=0.0045) and higher proportions of intensive care unit admissions (P=0.0003) and emergency department visits (P=0.0005).
Conclusion
These real-world data illustrate the differences in demographic and clinical characteristics, first-line treatment patterns, and HRU between FLT3mut+ and FLT3wt AML patient populations in Canada, the UK, France, Germany, Italy, and Spain. Age and FLT3 mutation status appeared to influence the type of induction regimen used; although most patients received HIC, FLT3mut+ vs FLT3wt patients were significantly younger and a higher percentage received HIC. Nearly half of FLT3mut+ patients receiving HIC also received a FLT3 inhibitor. The high use of supportive care and inpatient and outpatient HRU highlight the significant morbidity and economic burden associated with managing AML, the importance of knowing patients’ FLT3 mutation status, and the need to further understand how patients with FLT3mut+ AML may differ from other patients with AML.
Keyword(s): Mutation status