
Contributions
Abstract: PB1386
Type: Publication Only
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Chidamide is a histone deacetylase inhibitor (HDACi), selectively suppresses the activities of histone deacetylases 1, 2, 3, and 10. Cladribine, the deoxyadenosine analog, exerts its cytotoxic effect by being rapidly phosphorylated to the undegradable triphosphate (2-CDATP) by adenosine deaminase. The anti-leukemia effects of the two drugs have been reported. However, the synergistic therapeutic effects of the two drugs are still undetermined in acute myeloid leukemia (AML).
Aims
The relevant research that combine the cladribine with chidamide in AML has not been reported. The present resarch is aim to explore the synergistic therapeutic effects of the two drugs in AML and to provide a novel drug combination for relapse and refractory AML patients.
Methods
Cell Counting Kit-8 (CCK-8) cell proliferation assay, Annexin V apoptosis assay and Propidium Iodide cell cycle assay were performed in AML cell lines U937, THP-1, MV4-11, and KG-1 in presence of chidamide, cladribine, chidamide+ cladribine, and no-treatment control for 48h. Data were expressed as the mean ± standard deviation. T-tests statistical analysis was employed for determining the significant difference between groups. A synergistic effect was determined by the combination indices (CIs) with CompuSyn software.
Results
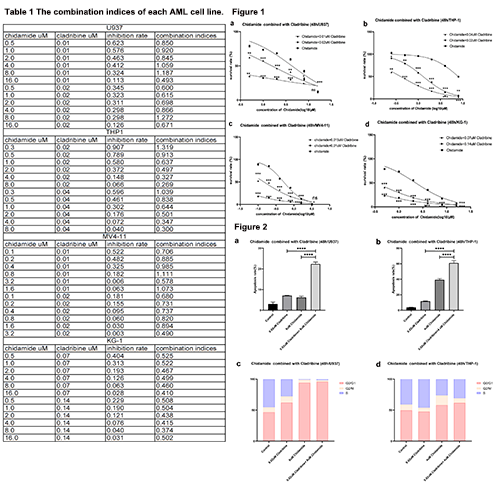
The 50% inhibiting concentration (IC50) of chidamide is 9.358±1.448μmol/L in U937 cells, 7.918±1.666μmol/L THP-1, 0.517±1.337μmol/L MV4-11, and 2.23±0.6885μmol/L KG-1. The IC50 of cladribine is 0.024±3.071μmol/L, 0.05±0.411μmol/L, 0.021±2.019μmol/L, and 0.14±3.13μmol/L in U937, THP-1, MV4-11, KG-1 cells, respectively. Two doses (IC50 or 1/2 IC50 ) of cladribine were respectively combined with various doses of chidamide for cell proliferation assay in each cell line. The survival rate decreases significantly in the combo group (chidamide plus cladribine) compared to single drug control in each cell line (Figure 1). The CI analysis showed that 7 combination doses of the two drugs have a significant synergistic effect in U937, and two in 8 in THP1, 8 in MV4-11, and all the tested combination doses (12) in KG-1 (Table 1). The combination of chidamide and cladribine significantly elevated the apoptosis rate of two cell lines, U937 and THP-1, compared with chidamide alone (p <0.0001) and cladribine alone (p <0.0001, Figure 2 a, b). The combo treatment also arrested the cell cycle at the G0/G1 phase in both THP-1 (p <0.0001) and U937 (p <0.0001, figure 2 c, d) cells.
Conclusion
Combination of chidamide and cladribine has a synergistic effect on cell proliferation arrest, apoptosis, and cell cycle arrest in acute myeloid leukemia cells. Our data provide experimental evidence for the novel potential combination on AML therapy. The clinical study is goning on.
Keyword(s): AML, Cladribine, HDAC inhibitor, Synergy
Abstract: PB1386
Type: Publication Only
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Chidamide is a histone deacetylase inhibitor (HDACi), selectively suppresses the activities of histone deacetylases 1, 2, 3, and 10. Cladribine, the deoxyadenosine analog, exerts its cytotoxic effect by being rapidly phosphorylated to the undegradable triphosphate (2-CDATP) by adenosine deaminase. The anti-leukemia effects of the two drugs have been reported. However, the synergistic therapeutic effects of the two drugs are still undetermined in acute myeloid leukemia (AML).
Aims
The relevant research that combine the cladribine with chidamide in AML has not been reported. The present resarch is aim to explore the synergistic therapeutic effects of the two drugs in AML and to provide a novel drug combination for relapse and refractory AML patients.
Methods
Cell Counting Kit-8 (CCK-8) cell proliferation assay, Annexin V apoptosis assay and Propidium Iodide cell cycle assay were performed in AML cell lines U937, THP-1, MV4-11, and KG-1 in presence of chidamide, cladribine, chidamide+ cladribine, and no-treatment control for 48h. Data were expressed as the mean ± standard deviation. T-tests statistical analysis was employed for determining the significant difference between groups. A synergistic effect was determined by the combination indices (CIs) with CompuSyn software.
Results
The 50% inhibiting concentration (IC50) of chidamide is 9.358±1.448μmol/L in U937 cells, 7.918±1.666μmol/L THP-1, 0.517±1.337μmol/L MV4-11, and 2.23±0.6885μmol/L KG-1. The IC50 of cladribine is 0.024±3.071μmol/L, 0.05±0.411μmol/L, 0.021±2.019μmol/L, and 0.14±3.13μmol/L in U937, THP-1, MV4-11, KG-1 cells, respectively. Two doses (IC50 or 1/2 IC50 ) of cladribine were respectively combined with various doses of chidamide for cell proliferation assay in each cell line. The survival rate decreases significantly in the combo group (chidamide plus cladribine) compared to single drug control in each cell line (Figure 1). The CI analysis showed that 7 combination doses of the two drugs have a significant synergistic effect in U937, and two in 8 in THP1, 8 in MV4-11, and all the tested combination doses (12) in KG-1 (Table 1). The combination of chidamide and cladribine significantly elevated the apoptosis rate of two cell lines, U937 and THP-1, compared with chidamide alone (p <0.0001) and cladribine alone (p <0.0001, Figure 2 a, b). The combo treatment also arrested the cell cycle at the G0/G1 phase in both THP-1 (p <0.0001) and U937 (p <0.0001, figure 2 c, d) cells.

Conclusion
Combination of chidamide and cladribine has a synergistic effect on cell proliferation arrest, apoptosis, and cell cycle arrest in acute myeloid leukemia cells. Our data provide experimental evidence for the novel potential combination on AML therapy. The clinical study is goning on.
Keyword(s): AML, Cladribine, HDAC inhibitor, Synergy


