
Contributions
Abstract: PB1381
Type: Publication Only
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Increased HOXA expression is associated with intermediate to poor prognosis in acute myeloid leukaemia (AML) and decreased expression acts as a readout for candidate experimental therapies. Underlying causes of increased HOXA expression range from gross rearrangements involving the Mixed Lineage Leukaemia (MLL) gene to mutations in single genes such as Nucleophosmin. To date, direct targeting of HOXA proteins has not been clinically feasible. In a Hoxa cluster-dependency model of MLL-rearranged leukaemia, we recently identified a group of FDA-approved drugs that could potentially target HOXA signatures indirectly by disrupting key pathways and processes.
Aims
This study aims to further identify and validate a subset of FDA-approved drugs in HOXA overexpressing AML.
Methods
RNA-sequencing was performed following conditional deletion of the Hoxa cluster in a mouse model of MLL-AF9 leukaemia. Bioinformatics tools including QUADrATiC, Ingenuity Pathway Analysis and the LINCS platform were used to identify candidate pathways and FDA-approved drugs. A subset of drugs were validated using cell viability assays in a panel of AML cell lines (including HL60, MOLM-13, MV4-11, OCI-AML3, THP1 and KG1a), and calculated IC50 values were obtained from dose-response curves for a subset of the candidate drugs.
Results
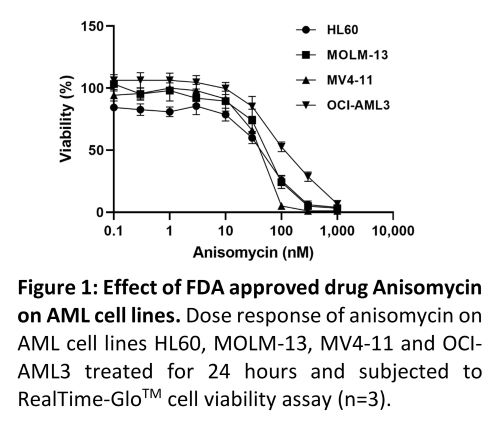
Connectivity mapping of the RNAseq data identified a list of candidate FDA-approved drugs with the potential to mimic HOXA deletion. These drugs include: Anisomycin, Butein, Calmidazolium, Digitoxigenin, Strophanthidin, Calcitriol, Emetine, Mefloquine and Trichostatin A. Cell viability assays identified measurable anti-leukaemia effects of some of these drugs at concentrations achievable clinically, most notably Anisomycin (Figure.1). Current and future work will evaluate anti-leukaemia effects of these drugs using colony forming ability, cell cycle and apoptosis analysis which will be presented during the meeting.
Conclusion
Drug repurposing is an attractive and economical approach to identifying new treatment options for AML patients. This study demonstrates anti-leukaemia activity of several drugs currently used for other diseases or disorders. It is anticipated that once fully validated, some of these drugs may be accelerated to clinical application, as the toxicity profiles are already established.
In summary, this study has identified and evaluated the potential of candidate FDA-approved drugs to induce cell death in AML cell lines. Further investigation of these drugs alone and in combination with first-line therapies will provide new and safer treatment options for AML patients.
Keyword(s): Acute myeloid leukemia, Drug resistance, HOX genes, Mixed lineage leukemia
Abstract: PB1381
Type: Publication Only
Session title: Acute myeloid leukemia - Biology & Translational Research
Background
Increased HOXA expression is associated with intermediate to poor prognosis in acute myeloid leukaemia (AML) and decreased expression acts as a readout for candidate experimental therapies. Underlying causes of increased HOXA expression range from gross rearrangements involving the Mixed Lineage Leukaemia (MLL) gene to mutations in single genes such as Nucleophosmin. To date, direct targeting of HOXA proteins has not been clinically feasible. In a Hoxa cluster-dependency model of MLL-rearranged leukaemia, we recently identified a group of FDA-approved drugs that could potentially target HOXA signatures indirectly by disrupting key pathways and processes.
Aims
This study aims to further identify and validate a subset of FDA-approved drugs in HOXA overexpressing AML.
Methods
RNA-sequencing was performed following conditional deletion of the Hoxa cluster in a mouse model of MLL-AF9 leukaemia. Bioinformatics tools including QUADrATiC, Ingenuity Pathway Analysis and the LINCS platform were used to identify candidate pathways and FDA-approved drugs. A subset of drugs were validated using cell viability assays in a panel of AML cell lines (including HL60, MOLM-13, MV4-11, OCI-AML3, THP1 and KG1a), and calculated IC50 values were obtained from dose-response curves for a subset of the candidate drugs.
Results
Connectivity mapping of the RNAseq data identified a list of candidate FDA-approved drugs with the potential to mimic HOXA deletion. These drugs include: Anisomycin, Butein, Calmidazolium, Digitoxigenin, Strophanthidin, Calcitriol, Emetine, Mefloquine and Trichostatin A. Cell viability assays identified measurable anti-leukaemia effects of some of these drugs at concentrations achievable clinically, most notably Anisomycin (Figure.1). Current and future work will evaluate anti-leukaemia effects of these drugs using colony forming ability, cell cycle and apoptosis analysis which will be presented during the meeting.

Conclusion
Drug repurposing is an attractive and economical approach to identifying new treatment options for AML patients. This study demonstrates anti-leukaemia activity of several drugs currently used for other diseases or disorders. It is anticipated that once fully validated, some of these drugs may be accelerated to clinical application, as the toxicity profiles are already established.
In summary, this study has identified and evaluated the potential of candidate FDA-approved drugs to induce cell death in AML cell lines. Further investigation of these drugs alone and in combination with first-line therapies will provide new and safer treatment options for AML patients.
Keyword(s): Acute myeloid leukemia, Drug resistance, HOX genes, Mixed lineage leukemia


