
Contributions
Abstract: PB1365
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Clinical
Background
Hypersensitivity is the most common side effect of L-Asparaginase (L-Asp), which is used in the treatment of acute lymphoblastic leukemia (ALL), most common malignancy of childhood with a survival rate of up to 90%. The irritating formulation is discontinued and replaced with other formulations, due to allergic reactions. Also, anti-asparaginase antibodies in patients who develop hypersensitivity may lead to decrease in drug efficacy, relapse and survival disadvantage.
Aims
In this study, we aimed to demonstrate the adverse effect of L-Asp hypersensitivity on the survival of children with newly diagnosed ALL by evaluating the survival of patients with and without L-Asp hypersensitivity comparatively.
Methods
182 newly diagnosed ALL patients whose mean age at diagnosis was 6.7 ± 4.4 years (1-18 years) treated with Modified St. Jude Total XV Therapy between 1 January 2008 and 30 January 2016. The survival of patients who showed a clinical allergic reaction to L-Asparaginase (Group A) and those who did not (Group B) were comparatively evaluatedretrospectively. Besides, in order to evaluate the effectiveness of High Dose Methylprednisolone (HDMP) treatment, patients were randomized consecutively and HDMP (maximum 1000 mg / day) of 20 mg/kg/day (n = 65) and 10 mg/kg/day (n = 90) were administered for 7 days prior to Modified St. Jude Total XV protocol induction therapy. MP at 10 mg/kg/day and 5 mg/kg/day were continued with induction therapy for initial 7 days, respectively. Then, MP at 2 mg/kg/day in both groups during day 8-21 of induction was given and discontinued. In patients with hyperleukocytosis at diagnosis (n=28), MP was started at lower doses (1 mg/kg/day) and gradually increased for 7 days.
Results
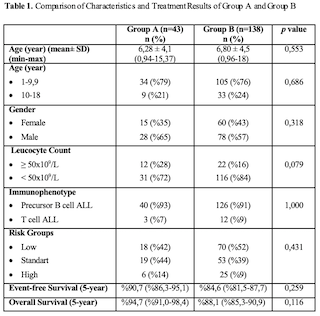
Clinical hypersensitivity reactions to L-Asp were observed in 43 (23%) patients (Group A), 40 (93%) during the maintenance and 3 (7%) during the induction, and desensitization was applied to the patients with antihistamine and steroid. The remaining 138 (%76) patients (Group B) did not show any clinical allergic reactions to L-Asp. The age, gender, leukocyte count, immunophenotype and risk group distribution at diagnosis of Group A and Group B were found to be similar (p = 0.553, p = 0.318, p = 0.079, p = 1,000, p = 0.431, respectively). In addition, no statistically significant difference was found between the rates of patients who developed L-Asp allergy in the two groups in which 10 mg/kg/day and 20 mg/kg/day YDMP treatment was initiated (p = 0.127). The 5-year event-free (EFS) and overall survival (OAS) rates of Group A and Group B were similar (p = 0.116 and p = 0.250, respectively). Although premedication was applied for subsequent doses, 21 (49%) of the patients with recurrent allergic reactions continued their treatment with PEG-Asparaginase and 16 (37%) with Erwinia L-Asp.
Conclusion
The incidence of allergic reaction, which is the most common side effect of L-Asp, has been reported in the literature in a wide range of 0-76% and mostly in the post-induction period. The rate of clinical allergic reaction to L-Asp found in our study was much lower compared to the St. Jude Total XV protocol (23% vs 40%). The observation of allergy in very few patients during induction can be explained by the suppression of hypersensitivity reaction due to the use of HDMP before and during the induction period. Additionally, no significant difference in the survival rates of patients who developed antibodies or allergies compared to patients who did not was revealed in accordance with our study.
Keyword(s): Acute lymphoblastic leukemia, Children, L-asparaginase, Survival
Abstract: PB1365
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Clinical
Background
Hypersensitivity is the most common side effect of L-Asparaginase (L-Asp), which is used in the treatment of acute lymphoblastic leukemia (ALL), most common malignancy of childhood with a survival rate of up to 90%. The irritating formulation is discontinued and replaced with other formulations, due to allergic reactions. Also, anti-asparaginase antibodies in patients who develop hypersensitivity may lead to decrease in drug efficacy, relapse and survival disadvantage.
Aims
In this study, we aimed to demonstrate the adverse effect of L-Asp hypersensitivity on the survival of children with newly diagnosed ALL by evaluating the survival of patients with and without L-Asp hypersensitivity comparatively.
Methods
182 newly diagnosed ALL patients whose mean age at diagnosis was 6.7 ± 4.4 years (1-18 years) treated with Modified St. Jude Total XV Therapy between 1 January 2008 and 30 January 2016. The survival of patients who showed a clinical allergic reaction to L-Asparaginase (Group A) and those who did not (Group B) were comparatively evaluatedretrospectively. Besides, in order to evaluate the effectiveness of High Dose Methylprednisolone (HDMP) treatment, patients were randomized consecutively and HDMP (maximum 1000 mg / day) of 20 mg/kg/day (n = 65) and 10 mg/kg/day (n = 90) were administered for 7 days prior to Modified St. Jude Total XV protocol induction therapy. MP at 10 mg/kg/day and 5 mg/kg/day were continued with induction therapy for initial 7 days, respectively. Then, MP at 2 mg/kg/day in both groups during day 8-21 of induction was given and discontinued. In patients with hyperleukocytosis at diagnosis (n=28), MP was started at lower doses (1 mg/kg/day) and gradually increased for 7 days.
Results
Clinical hypersensitivity reactions to L-Asp were observed in 43 (23%) patients (Group A), 40 (93%) during the maintenance and 3 (7%) during the induction, and desensitization was applied to the patients with antihistamine and steroid. The remaining 138 (%76) patients (Group B) did not show any clinical allergic reactions to L-Asp. The age, gender, leukocyte count, immunophenotype and risk group distribution at diagnosis of Group A and Group B were found to be similar (p = 0.553, p = 0.318, p = 0.079, p = 1,000, p = 0.431, respectively). In addition, no statistically significant difference was found between the rates of patients who developed L-Asp allergy in the two groups in which 10 mg/kg/day and 20 mg/kg/day YDMP treatment was initiated (p = 0.127). The 5-year event-free (EFS) and overall survival (OAS) rates of Group A and Group B were similar (p = 0.116 and p = 0.250, respectively). Although premedication was applied for subsequent doses, 21 (49%) of the patients with recurrent allergic reactions continued their treatment with PEG-Asparaginase and 16 (37%) with Erwinia L-Asp.

Conclusion
The incidence of allergic reaction, which is the most common side effect of L-Asp, has been reported in the literature in a wide range of 0-76% and mostly in the post-induction period. The rate of clinical allergic reaction to L-Asp found in our study was much lower compared to the St. Jude Total XV protocol (23% vs 40%). The observation of allergy in very few patients during induction can be explained by the suppression of hypersensitivity reaction due to the use of HDMP before and during the induction period. Additionally, no significant difference in the survival rates of patients who developed antibodies or allergies compared to patients who did not was revealed in accordance with our study.
Keyword(s): Acute lymphoblastic leukemia, Children, L-asparaginase, Survival


