
Contributions
Abstract: PB1361
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Clinical
Background
Loss of patient-specific HLA after haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is considered as a relapse mechanism for lacking the incompatible molecule to elicit alloreactivity, which extensively diminishing graft versus leukemia (GVL) effects. Blinatumomab, as a CD3/CD19 bispecific antibody, can yield a profound response via redirecting T cells towards malignant lymphoblasts in B-cell acute lymphoblastic leukemia (B-ALL). We aimed to assess whether blinatumomab could result in a considerable response by regenerating immunological engagement in patients with HLA loss relapse after haplo-HSCT.
Aims
As there are no clinical data on blinatumomab for posttransplantation HLA loss relapse, we herein report the preliminary results of blinatumomab in treating B-ALL with HLA loss recurrence after haplo-HSCT.
Methods
We recruited patients with B-ALL at the First Affiliated Hospital of Zhejiang University School of Medicine. Eligible patients were diagnosed with HLA loss relapse after haplo-HSCT. One cycle of blinatumomab treatment lasted for 6 weeks, including a 4-week continuous i.v. infusion period and a 2-week treatment-free interval. During the first induction cycle, an initial dose of 9 μg/day was used for the first 7 days and the dose was increased to 28 μg/day starting on day 8 (week 2) and continuing through day 29 (week 4). The 28 μg/day dose was used for all subsequent cycles. Patients received up to 5 cycles of blinatumomab, with efficacy follow-up at 3, 6, 9, 12, 18, and 24 months after treatment initiation. The primary endpoint was CR/CRh rates in the 2 cycles of blinatumomab treatment.
Results
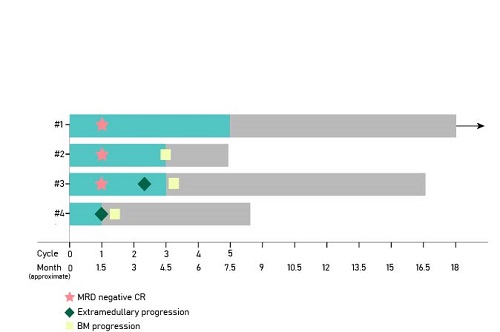
Between January 2017 and July 2019, 4 eligible patients relapsed HLA loss relapse after haplo-HSCT were enrolled in the study. Four patients achieved a CR/CRh with three MRD-negative response within the first 1 cycle of treatment. Three of the four met a primary endpoint with CR/CRh and MRD-negative response within 2 cycles of treatment. One patient developed new EM sites of skin after the first cycle (Fig 1). Cytokine release syndrome was observed in one patient. Cytopenias, as well as elevated alanine aminotransferase and aspartate aminotransferase, were two common adverse effects during treatment.
Conclusion
Blinatumomab can induce rapid and robust remission in B-ALL patients undergoing HLA loss relapsed after haplo-HSCT. By redirecting lysis of CD19-positive lymphoblast who losing the incompatible HLA, blinatumomab is a potential strategy to eradicate malignant cells via restoring GVL effects. A randomized clinical trial assessing blinatumomab in patients with HLA loss relapse after HSCT is warranted.
Keyword(s): B cell acute lymphoblastic leukemia, Bispecific, HLA, Relapsed acute lymphoblastic leukemia
Abstract: PB1361
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Clinical
Background
Loss of patient-specific HLA after haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is considered as a relapse mechanism for lacking the incompatible molecule to elicit alloreactivity, which extensively diminishing graft versus leukemia (GVL) effects. Blinatumomab, as a CD3/CD19 bispecific antibody, can yield a profound response via redirecting T cells towards malignant lymphoblasts in B-cell acute lymphoblastic leukemia (B-ALL). We aimed to assess whether blinatumomab could result in a considerable response by regenerating immunological engagement in patients with HLA loss relapse after haplo-HSCT.
Aims
As there are no clinical data on blinatumomab for posttransplantation HLA loss relapse, we herein report the preliminary results of blinatumomab in treating B-ALL with HLA loss recurrence after haplo-HSCT.
Methods
We recruited patients with B-ALL at the First Affiliated Hospital of Zhejiang University School of Medicine. Eligible patients were diagnosed with HLA loss relapse after haplo-HSCT. One cycle of blinatumomab treatment lasted for 6 weeks, including a 4-week continuous i.v. infusion period and a 2-week treatment-free interval. During the first induction cycle, an initial dose of 9 μg/day was used for the first 7 days and the dose was increased to 28 μg/day starting on day 8 (week 2) and continuing through day 29 (week 4). The 28 μg/day dose was used for all subsequent cycles. Patients received up to 5 cycles of blinatumomab, with efficacy follow-up at 3, 6, 9, 12, 18, and 24 months after treatment initiation. The primary endpoint was CR/CRh rates in the 2 cycles of blinatumomab treatment.
Results
Between January 2017 and July 2019, 4 eligible patients relapsed HLA loss relapse after haplo-HSCT were enrolled in the study. Four patients achieved a CR/CRh with three MRD-negative response within the first 1 cycle of treatment. Three of the four met a primary endpoint with CR/CRh and MRD-negative response within 2 cycles of treatment. One patient developed new EM sites of skin after the first cycle (Fig 1). Cytokine release syndrome was observed in one patient. Cytopenias, as well as elevated alanine aminotransferase and aspartate aminotransferase, were two common adverse effects during treatment.

Conclusion
Blinatumomab can induce rapid and robust remission in B-ALL patients undergoing HLA loss relapsed after haplo-HSCT. By redirecting lysis of CD19-positive lymphoblast who losing the incompatible HLA, blinatumomab is a potential strategy to eradicate malignant cells via restoring GVL effects. A randomized clinical trial assessing blinatumomab in patients with HLA loss relapse after HSCT is warranted.
Keyword(s): B cell acute lymphoblastic leukemia, Bispecific, HLA, Relapsed acute lymphoblastic leukemia


