Contributions
Abstract: PB1355
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Currently, no International Standards are available for the quantitation of the e1a2 p190 transcript for both CML and ALL, posing a challenge when developing a quantitative assay to monitor the e1a2 p190 transcript levels.
Aims
Implementation of a primary control material, containing in vitro transcribed RNA (IVT-RNA) of ABL and BCR-ABL e1a2, for standardization of ABL and BCR-ABL e1a2 quantifications through copy number (CN) calibration was used to develop an assay calibration methodology at Cepheid. This process allows generation of in-house RNA control materials as secondary standard (SS) mimicking clinical samples, for calibration and standardization of the Xpert BCR-ABL Ultra p190 Prototype Assay.
Methods
Titration studies were performed with Cepheid’s IVT-RNA, containing e1a2 and ABL, to derive CN calibration curves of the target genes relative to the corresponding Cycle Threshold (Ct) for both e1a2 and ABL in the background of PAXgene human whole blood. The calibrated standard curves assigned the BCR-ABL e1a2/ABL% values to a 4-member in-house control material (SS) ranging from ~10% to 0.02% generated by spiking BCR-ABL e1a2 total RNA and ABL total RNA (HL60) in PAXgene blood. A lot-specific curve of delta Ct vs BCR-ABL e1a2/ABL% value was generated from the secondary standard for the BCR-ABL p190 Prototype Assay providing an Efficiency Value (E?Ct) and a lot-specific scaling factor (SF). The BCR-ABL e1a2/ABL% value of any given clinical sample was calculated with E?Ct and SF. A 4-assay comparison study was performed to validate the assay calibration methodology with contrived clinical samples.
Results
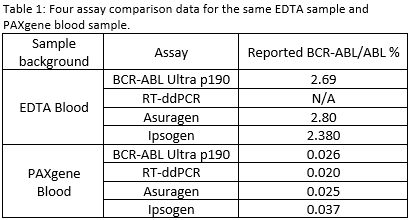
Based on the observation of constant delta Ct between e1a2 and ABL from IVT-RNA, the ABL standard curve was generated by BCR-ABL e1a2 standard curve. Through the BCR-ABL e1a2 and ABL calibration curves generated from IVT-RNA PAXgene blood background, the BCR-ABL e1a2/ABL % value of the SS was assigned. The E?Ct and SF of 4 lots of BCR-ABL Ultra p190 kit were generated. BCR-ABL e1a2/ABL % values of contrived clinical samples in EDTA whole blood and PAXgene whole blood from BCR-ABL Ultra p190 Prototype Assay were consistent with those from three other comparator assays, RT-ddPCR, Asuragen, and Ipsogen (Table 1).
Conclusion
In conclusion, implementing a primary control material of IVT-RNA, allowed linkage of known RNA CN to corresponding Ct, thus providing a methodology to assign BCR-ABL e1a2/ABL % value to a human whole blood-based secondary standard. A lot-specific E?Ct and SF for BCR-ABL Ultra p190 Prototype Assay was generated from the secondary standard and was utilized to calculate the BCR-ABL e1a2/ABL % value for a given clinical sample. Data from 4-assay comparison suggested that we achieved the goal of establishing a successful assay calibration methodology with IVT-RNA in human blood background as primary standard and the BCR-ABL e1a2/ABL % value reported by BCR-ABL Ultra p190 Prototype Assay was compatible with other comparator assays.
Keyword(s): Acute lymphoblastic leukemia, Assay
Abstract: PB1355
Type: Publication Only
Session title: Acute lymphoblastic leukemia - Biology & Translational Research
Background
Currently, no International Standards are available for the quantitation of the e1a2 p190 transcript for both CML and ALL, posing a challenge when developing a quantitative assay to monitor the e1a2 p190 transcript levels.
Aims
Implementation of a primary control material, containing in vitro transcribed RNA (IVT-RNA) of ABL and BCR-ABL e1a2, for standardization of ABL and BCR-ABL e1a2 quantifications through copy number (CN) calibration was used to develop an assay calibration methodology at Cepheid. This process allows generation of in-house RNA control materials as secondary standard (SS) mimicking clinical samples, for calibration and standardization of the Xpert BCR-ABL Ultra p190 Prototype Assay.
Methods
Titration studies were performed with Cepheid’s IVT-RNA, containing e1a2 and ABL, to derive CN calibration curves of the target genes relative to the corresponding Cycle Threshold (Ct) for both e1a2 and ABL in the background of PAXgene human whole blood. The calibrated standard curves assigned the BCR-ABL e1a2/ABL% values to a 4-member in-house control material (SS) ranging from ~10% to 0.02% generated by spiking BCR-ABL e1a2 total RNA and ABL total RNA (HL60) in PAXgene blood. A lot-specific curve of delta Ct vs BCR-ABL e1a2/ABL% value was generated from the secondary standard for the BCR-ABL p190 Prototype Assay providing an Efficiency Value (E?Ct) and a lot-specific scaling factor (SF). The BCR-ABL e1a2/ABL% value of any given clinical sample was calculated with E?Ct and SF. A 4-assay comparison study was performed to validate the assay calibration methodology with contrived clinical samples.
Results
Based on the observation of constant delta Ct between e1a2 and ABL from IVT-RNA, the ABL standard curve was generated by BCR-ABL e1a2 standard curve. Through the BCR-ABL e1a2 and ABL calibration curves generated from IVT-RNA PAXgene blood background, the BCR-ABL e1a2/ABL % value of the SS was assigned. The E?Ct and SF of 4 lots of BCR-ABL Ultra p190 kit were generated. BCR-ABL e1a2/ABL % values of contrived clinical samples in EDTA whole blood and PAXgene whole blood from BCR-ABL Ultra p190 Prototype Assay were consistent with those from three other comparator assays, RT-ddPCR, Asuragen, and Ipsogen (Table 1).
Conclusion
In conclusion, implementing a primary control material of IVT-RNA, allowed linkage of known RNA CN to corresponding Ct, thus providing a methodology to assign BCR-ABL e1a2/ABL % value to a human whole blood-based secondary standard. A lot-specific E?Ct and SF for BCR-ABL Ultra p190 Prototype Assay was generated from the secondary standard and was utilized to calculate the BCR-ABL e1a2/ABL % value for a given clinical sample. Data from 4-assay comparison suggested that we achieved the goal of establishing a successful assay calibration methodology with IVT-RNA in human blood background as primary standard and the BCR-ABL e1a2/ABL % value reported by BCR-ABL Ultra p190 Prototype Assay was compatible with other comparator assays.
Keyword(s): Acute lymphoblastic leukemia, Assay