Contributions
Abstract: EP955
Type: e-Poster
Background
Belantamab mafodotin (belamaf; GSK2857916) is an investigational first-in-class B-cell maturation antigen-binding, humanized, afucosylated, monoclonal immunoconjugate. The pivotal DREAMM-2 study demonstrated that single-agent belamaf had clinically meaningful activity and a manageable safety profile in patients with heavily pretreated RRMM (DREAMM-2, NCT03525678, Lancet Oncol. 2020). Immune surveillance in patients with multiple myeloma (MM) is often blunted through the PD-1/PDL-1 axis, whereby PDL-1 is highly expressed on MM cells. Pembrolizumab, a PD-1 inhibitor, could augment immune responses to belamaf-induced immunogenic cell death (one of the multimodal belamaf mechanisms of action). We have designed a study to evaluate the safety and efficacy of belamaf and pembrolizumab in combination in RRMM.
Aims
DREAMM-4 (NCT03848845) is an ongoing Phase I/II, single-arm, open-label, two-part study to assess the safety, tolerability, clinical activity, and other outcomes in this combination study in RRMM.
Methods
All patients provided informed consent and had ≥3 prior lines of therapy, including anti-CD38 treatment. Planned enrollment for both parts is up to 40 patients. Part 1 (dose escalation) is evaluating 2 doses of belamaf (2.5 or 3.4 mg/kg) plus 200 mg pembrolizumab in up to 12 patients to establish the recommended Phase II dose (RP2D) of this combination (dose escalation decisions guided by modified toxicity probability interval). Both drugs are administered intravenously Q3W for up to 35 cycles. Part 1 interim analyses are presented. Part 2 (dose expansion) will confirm the safety profile and explore the clinical activity at the RP2D with the overall response rate (ORR; ≥partial response) as the primary endpoint.
Results
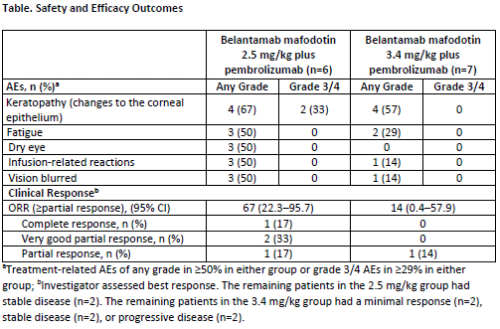
As of 04 Feb 2020 (interim analysis [IA] data cut-off), 6 patients were enrolled at the 2.5 mg/kg belamaf dose and 7 were enrolled at the 3.4 mg/kg dose (1 patient replaced as per protocol). In the 2.5 mg/kg and 3.4 mg/kg groups, respectively, the median (range) numbers of prior lines of therapy were 7.5 (3–13) and 5.0 (3–8), and 3/6 (50%) and 1/7 (14%) patients had high-risk cytogenetics. No dose limiting toxicities have been reported in either group. All patients in both groups experienced AEs and treatment-related AEs. The most frequent AEs of any grade and Grade 3/4 are reported in the Table. Serious AEs (SAEs) were reported in 50% (3/6) of patients in the 2.5 mg/kg group (1 patient had a treatment-related SAE) and 43% (3/7) of patients in the 3.4 mg/kg group (3 patients had a treatment-related SAE). No Grade 5 AEs occurred. Keratopathy (changes to the corneal epithelium) was the primary reason for dose delays (2.5 mg/kg group: 67%; 3.4 mg/kg group: 14%) and dose reductions (2.5 mg/kg group: 17%; 3.4 mg/kg group: 0%). The ORR was 67% in the 2.5 mg/kg group and 14% in the 3.4 mg/kg group (Table). PK data are pending. Free soluble BCMA concentration changes were consistent with those in DREAMM-1 (NCT02064387).
Conclusion
Belamaf plus pembrolizumab demonstrated a manageable safety profile and clinical activity in patients with RRMM. Changes to the corneal epithelium were the most frequent AEs, consistent with previous reports from belamaf studies, and were managed with dose modifications. The Part 1 IA supports continuation with Part 2 of this study.
Funding: GlaxoSmithKline (205207) in collaboration with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Drug linker technology licensed from Seattle Genetics; monoclonal antibody produced using POTELLIGENT technology licensed from BioWa.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Clinical trial, Immunoconjugate, Multiple myeloma
Abstract: EP955
Type: e-Poster
Background
Belantamab mafodotin (belamaf; GSK2857916) is an investigational first-in-class B-cell maturation antigen-binding, humanized, afucosylated, monoclonal immunoconjugate. The pivotal DREAMM-2 study demonstrated that single-agent belamaf had clinically meaningful activity and a manageable safety profile in patients with heavily pretreated RRMM (DREAMM-2, NCT03525678, Lancet Oncol. 2020). Immune surveillance in patients with multiple myeloma (MM) is often blunted through the PD-1/PDL-1 axis, whereby PDL-1 is highly expressed on MM cells. Pembrolizumab, a PD-1 inhibitor, could augment immune responses to belamaf-induced immunogenic cell death (one of the multimodal belamaf mechanisms of action). We have designed a study to evaluate the safety and efficacy of belamaf and pembrolizumab in combination in RRMM.
Aims
DREAMM-4 (NCT03848845) is an ongoing Phase I/II, single-arm, open-label, two-part study to assess the safety, tolerability, clinical activity, and other outcomes in this combination study in RRMM.
Methods
All patients provided informed consent and had ≥3 prior lines of therapy, including anti-CD38 treatment. Planned enrollment for both parts is up to 40 patients. Part 1 (dose escalation) is evaluating 2 doses of belamaf (2.5 or 3.4 mg/kg) plus 200 mg pembrolizumab in up to 12 patients to establish the recommended Phase II dose (RP2D) of this combination (dose escalation decisions guided by modified toxicity probability interval). Both drugs are administered intravenously Q3W for up to 35 cycles. Part 1 interim analyses are presented. Part 2 (dose expansion) will confirm the safety profile and explore the clinical activity at the RP2D with the overall response rate (ORR; ≥partial response) as the primary endpoint.
Results
As of 04 Feb 2020 (interim analysis [IA] data cut-off), 6 patients were enrolled at the 2.5 mg/kg belamaf dose and 7 were enrolled at the 3.4 mg/kg dose (1 patient replaced as per protocol). In the 2.5 mg/kg and 3.4 mg/kg groups, respectively, the median (range) numbers of prior lines of therapy were 7.5 (3–13) and 5.0 (3–8), and 3/6 (50%) and 1/7 (14%) patients had high-risk cytogenetics. No dose limiting toxicities have been reported in either group. All patients in both groups experienced AEs and treatment-related AEs. The most frequent AEs of any grade and Grade 3/4 are reported in the Table. Serious AEs (SAEs) were reported in 50% (3/6) of patients in the 2.5 mg/kg group (1 patient had a treatment-related SAE) and 43% (3/7) of patients in the 3.4 mg/kg group (3 patients had a treatment-related SAE). No Grade 5 AEs occurred. Keratopathy (changes to the corneal epithelium) was the primary reason for dose delays (2.5 mg/kg group: 67%; 3.4 mg/kg group: 14%) and dose reductions (2.5 mg/kg group: 17%; 3.4 mg/kg group: 0%). The ORR was 67% in the 2.5 mg/kg group and 14% in the 3.4 mg/kg group (Table). PK data are pending. Free soluble BCMA concentration changes were consistent with those in DREAMM-1 (NCT02064387).
Conclusion
Belamaf plus pembrolizumab demonstrated a manageable safety profile and clinical activity in patients with RRMM. Changes to the corneal epithelium were the most frequent AEs, consistent with previous reports from belamaf studies, and were managed with dose modifications. The Part 1 IA supports continuation with Part 2 of this study.
Funding: GlaxoSmithKline (205207) in collaboration with Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Drug linker technology licensed from Seattle Genetics; monoclonal antibody produced using POTELLIGENT technology licensed from BioWa.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Clinical trial, Immunoconjugate, Multiple myeloma