
Contributions
Abstract: LB2603
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 11:45 - 12:00
Location: Room A1
Background
Ravulizumab, an innovative C5 inhibitor with high C5 affinity and half-life 3-4 times longer than eculizumab, provides immediate, complete, and sustained C5 inhibition with extended dosing intervals.
Aims
To compare efficacy and safety of ravulizumab vs eculizumab in adult patients (pts) with PNH naive to complement inhibitor therapy (NCT02946463).
Methods
In this phase 3, randomized, open-label, noninferiority, multicenter study, complement inhibitor-naive PNH pts with ≥1 PNH-related sign/symptom and LDH level ≥1.5 xULN at screening were stratified by transfusion history (0, 1-14, or >14 units of red blood cells 1 yr before study) and screening LDH level (1.5-<3 xULN or ≥3 xULN), and randomized 1:1 to receive ravulizumab (loading/maintenance dose [day 1/day 15 and q8w thereafter] as follows: ≥40-<60 kg body weight [2400/3000 mg]; ≥60-<100 kg [2700/3300 mg]; ≥100 kg [3000/3600 mg]), or eculizumab at approved dosing regimen through day 183 (primary evaluation period). Primary efficacy endpoints included transfusion avoidance (TA; proportion of pts who remain transfusion-free) and LDH normalization (LDH ULN=246 U/L) from days 29–183. Noninferiority was declared if the lower bound of the 95% CI for difference in proportion of pts with TA receiving ravulizumab vs eculizumab was >–20% and lower bound of the 95% CI for odds ratio for LDH normalization between ravulizumab vs eculizumab was >0.39. Key secondary endpoints were % change in LDH from baseline (BL), change from BL in FACIT-Fatigue score, and proportions of pts with breakthrough hemolysis (BTH) and stabilized hemoglobin (Hb). Pharmacodynamic effects were measured by change in free C5.
Results
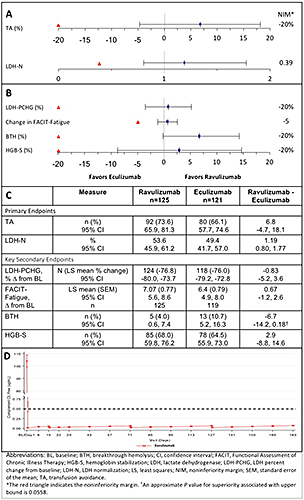
Of 285 pts screened, 246 from countries across the Asia-Pacific region, Europe, and North and South America were randomized. All 125 ravulizumab pts and 119/121 eculizumab pts completed 26 wks of treatment. Ravulizumab met the primary objective of statistically significant noninferiority vs eculizumab on both primary endpoints and all 4 key secondary endpoints, with trends favoring ravulizumab over eculizumab for all 6 endpoints. Ravulizumab was noninferior to eculizumab in proportions of pts achieving TA (73.6% vs 66.1%; difference, 6.8% [95% CI, -4.7, 18.1%]) and LDH normalization (53.6% vs 49.4%, OR 1.19 [0.80, 1.77]) (Fig A&C). Point estimates favored ravulizumab in % change in least squares mean LDH levels (76.8% vs 76.0% reduction from BL), change in FACIT-Fatigue score (7.1 vs 6.4 point improvement from BL), and % of pts with BTH (4.0% vs 10.7%) and Hb stabilization (68.0% vs 64.5%) (Fig B&C). Immediate, complete, and sustained inhibition of C5 (mean free C5 <0.5 μg/mL) was observed by end of first infusion of ravulizumab (Fig D). Most frequently reported treatment-emergent adverse event (AE) was headache (36.0%/33.1% in pts receiving ravulizumab/eculizumab). Serious AEs were experienced by 8.8%/7.4% in the ravulizumab/eculizumab groups. Major adverse vascular events occurred in 3 pts (ravulizumab, 2; eculizumab, 1). There were no meningococcal infections.
Conclusion
In the largest study of PNH pts, q8w ravulizumab achieved statistically significant noninferiority to q2w eculizumab on all primary and key secondary endpoints, with an efficacy profile consistent with immediate, complete, and sustained inhibition of free C5. Safety was similar in both groups.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): Complement, Monoclonal antibody, transfusion, Paroxysmal nocturnal hemoglobinuria (PNH)
Abstract: LB2603
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 11:45 - 12:00
Location: Room A1
Background
Ravulizumab, an innovative C5 inhibitor with high C5 affinity and half-life 3-4 times longer than eculizumab, provides immediate, complete, and sustained C5 inhibition with extended dosing intervals.
Aims
To compare efficacy and safety of ravulizumab vs eculizumab in adult patients (pts) with PNH naive to complement inhibitor therapy (NCT02946463).
Methods
In this phase 3, randomized, open-label, noninferiority, multicenter study, complement inhibitor-naive PNH pts with ≥1 PNH-related sign/symptom and LDH level ≥1.5 xULN at screening were stratified by transfusion history (0, 1-14, or >14 units of red blood cells 1 yr before study) and screening LDH level (1.5-<3 xULN or ≥3 xULN), and randomized 1:1 to receive ravulizumab (loading/maintenance dose [day 1/day 15 and q8w thereafter] as follows: ≥40-<60 kg body weight [2400/3000 mg]; ≥60-<100 kg [2700/3300 mg]; ≥100 kg [3000/3600 mg]), or eculizumab at approved dosing regimen through day 183 (primary evaluation period). Primary efficacy endpoints included transfusion avoidance (TA; proportion of pts who remain transfusion-free) and LDH normalization (LDH ULN=246 U/L) from days 29–183. Noninferiority was declared if the lower bound of the 95% CI for difference in proportion of pts with TA receiving ravulizumab vs eculizumab was >–20% and lower bound of the 95% CI for odds ratio for LDH normalization between ravulizumab vs eculizumab was >0.39. Key secondary endpoints were % change in LDH from baseline (BL), change from BL in FACIT-Fatigue score, and proportions of pts with breakthrough hemolysis (BTH) and stabilized hemoglobin (Hb). Pharmacodynamic effects were measured by change in free C5.
Results
Of 285 pts screened, 246 from countries across the Asia-Pacific region, Europe, and North and South America were randomized. All 125 ravulizumab pts and 119/121 eculizumab pts completed 26 wks of treatment. Ravulizumab met the primary objective of statistically significant noninferiority vs eculizumab on both primary endpoints and all 4 key secondary endpoints, with trends favoring ravulizumab over eculizumab for all 6 endpoints. Ravulizumab was noninferior to eculizumab in proportions of pts achieving TA (73.6% vs 66.1%; difference, 6.8% [95% CI, -4.7, 18.1%]) and LDH normalization (53.6% vs 49.4%, OR 1.19 [0.80, 1.77]) (Fig A&C). Point estimates favored ravulizumab in % change in least squares mean LDH levels (76.8% vs 76.0% reduction from BL), change in FACIT-Fatigue score (7.1 vs 6.4 point improvement from BL), and % of pts with BTH (4.0% vs 10.7%) and Hb stabilization (68.0% vs 64.5%) (Fig B&C). Immediate, complete, and sustained inhibition of C5 (mean free C5 <0.5 μg/mL) was observed by end of first infusion of ravulizumab (Fig D). Most frequently reported treatment-emergent adverse event (AE) was headache (36.0%/33.1% in pts receiving ravulizumab/eculizumab). Serious AEs were experienced by 8.8%/7.4% in the ravulizumab/eculizumab groups. Major adverse vascular events occurred in 3 pts (ravulizumab, 2; eculizumab, 1). There were no meningococcal infections.

Conclusion
In the largest study of PNH pts, q8w ravulizumab achieved statistically significant noninferiority to q2w eculizumab on all primary and key secondary endpoints, with an efficacy profile consistent with immediate, complete, and sustained inhibition of free C5. Safety was similar in both groups.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): Complement, Monoclonal antibody, transfusion, Paroxysmal nocturnal hemoglobinuria (PNH)


