
Contributions
Abstract: PB2377
Type: Publication Only
Background
Chemotherapy-induced nausea and vomiting (CINV) is a significant side effect during stem cell transplantation (SCT) despite prophylactic therapy. NEPA (Netupitant plus palonosetron) has proven to be highly effective in controlling these symptoms in patients with solid tumors; however, data are scarce in SCT recipients.
Aims
To evaluate the efficacy and safety of NEPA in two different cohorts of SCT patients.
Methods
Two cohorts of patients were analysed: those undergoing high emetogenic conditioning regimens before SCT and those receiving high doses of cyclophosphamide as graft vs. host disease (GVHD) prophylaxis.
On the first day of chemotherapy (CT), the cohort of patients receiving high emetogenic conditioning regimens (group number 1) were prescribed an oral fixed-dose combination of NEPA (300 mg netupitant, 0.5mg palonosetron) together with 8 mg of dexamethasone (DXM) followed by daily DXM at a dose of 8 mg whilst receiving CT and 4 mg in the subsequent 48 hours. On the other hand, the cohort of patients being prescribed GVHD prophylaxis based on high dose cyclophosphamide (group number 2), were given NEPA on day +3 after infusion (first day of cyclophosphamide) and DXM was not administered before day +5.
Results
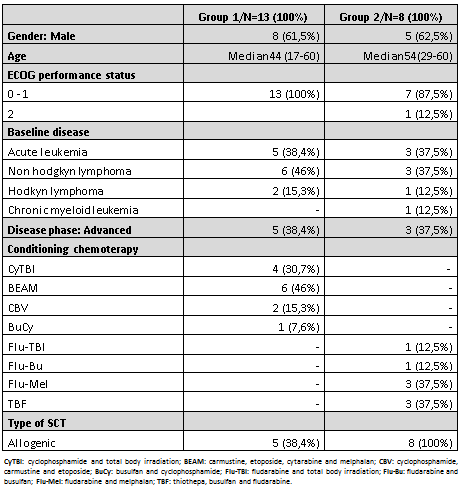
Twenty-one patients were included in the study: 13 in group number 1 and 8 in group number 2. Baseline characteristics are detailed in Table 1.
In regard to patients in group number 1, during the acute phase of treatment (days in which patients receive CT), 6 (46%) out of the 13 patients achieved complete response (CR, defined as no emesis or no need for antiemetic rescue) and 4 (31%) partial response (PR, defined as presence of mild to moderate nausea but no emesis). The maximum number of emetic episodes experienced per day was of 2 in two patients. Six patients required additional treatment for breakthrough emesis and were all successfully controlled with single standard doses of olanzapine (n=2), metoclopramide (n=3) or lorazepam (n=1). Responses improved during the delayed phase (up to 72 hours after the last dose of chemotherapy) where 9 patients (69%) achieved CR and 3 patients (23%) achieved PR.
In respect to patients in group number 2, during the acute phase (period of time whilst receiving cychophosphamide postSCT), 4 patients (57%) achieved CR and 5 patients (71%) PR. Four patients were successfully controlled for breakthrough emesis during this time with single standard doses of metoclopramide.
Regarding the delayed phase, 5 patients achieved CR (62.5%) and 4 (50%) PR. 4 patients required single standard doses of metoclopramide (n=2) and olanzapine (n=2) for breakthrough emesis with appropriate response.
Of the 21 patients evaluated, 11 (52%) had a history of significant nausea and emesis with prior chemotherapies, 7 (33, 3%) of which did not develop emesis with the NEPA regimen.
Non-hematologic adverse effects attributed to the study medications were minor (hiccups n=3, somnolence n=3, hyperglycemia n=10, constipation n=3); all of which gave no further complications.
Conclusion
NEPA-based antiemetic regimens seem to offer encouraging results in terms of prophylaxis of CINV in the SCT setting, including different procedures (autologous and allogeneic transplants) and new options such as Cy-post for GVHD prevention. Based on these promising results in terms of efficacy and safety, enrolment of new patients is currently ongoing.
Session topic: 36. Quality of life, palliative care, ethics and health economics
Abstract: PB2377
Type: Publication Only
Background
Chemotherapy-induced nausea and vomiting (CINV) is a significant side effect during stem cell transplantation (SCT) despite prophylactic therapy. NEPA (Netupitant plus palonosetron) has proven to be highly effective in controlling these symptoms in patients with solid tumors; however, data are scarce in SCT recipients.
Aims
To evaluate the efficacy and safety of NEPA in two different cohorts of SCT patients.
Methods
Two cohorts of patients were analysed: those undergoing high emetogenic conditioning regimens before SCT and those receiving high doses of cyclophosphamide as graft vs. host disease (GVHD) prophylaxis.
On the first day of chemotherapy (CT), the cohort of patients receiving high emetogenic conditioning regimens (group number 1) were prescribed an oral fixed-dose combination of NEPA (300 mg netupitant, 0.5mg palonosetron) together with 8 mg of dexamethasone (DXM) followed by daily DXM at a dose of 8 mg whilst receiving CT and 4 mg in the subsequent 48 hours. On the other hand, the cohort of patients being prescribed GVHD prophylaxis based on high dose cyclophosphamide (group number 2), were given NEPA on day +3 after infusion (first day of cyclophosphamide) and DXM was not administered before day +5.
Results
Twenty-one patients were included in the study: 13 in group number 1 and 8 in group number 2. Baseline characteristics are detailed in Table 1.
In regard to patients in group number 1, during the acute phase of treatment (days in which patients receive CT), 6 (46%) out of the 13 patients achieved complete response (CR, defined as no emesis or no need for antiemetic rescue) and 4 (31%) partial response (PR, defined as presence of mild to moderate nausea but no emesis). The maximum number of emetic episodes experienced per day was of 2 in two patients. Six patients required additional treatment for breakthrough emesis and were all successfully controlled with single standard doses of olanzapine (n=2), metoclopramide (n=3) or lorazepam (n=1). Responses improved during the delayed phase (up to 72 hours after the last dose of chemotherapy) where 9 patients (69%) achieved CR and 3 patients (23%) achieved PR.
In respect to patients in group number 2, during the acute phase (period of time whilst receiving cychophosphamide postSCT), 4 patients (57%) achieved CR and 5 patients (71%) PR. Four patients were successfully controlled for breakthrough emesis during this time with single standard doses of metoclopramide.
Regarding the delayed phase, 5 patients achieved CR (62.5%) and 4 (50%) PR. 4 patients required single standard doses of metoclopramide (n=2) and olanzapine (n=2) for breakthrough emesis with appropriate response.
Of the 21 patients evaluated, 11 (52%) had a history of significant nausea and emesis with prior chemotherapies, 7 (33, 3%) of which did not develop emesis with the NEPA regimen.
Non-hematologic adverse effects attributed to the study medications were minor (hiccups n=3, somnolence n=3, hyperglycemia n=10, constipation n=3); all of which gave no further complications.

Conclusion
NEPA-based antiemetic regimens seem to offer encouraging results in terms of prophylaxis of CINV in the SCT setting, including different procedures (autologous and allogeneic transplants) and new options such as Cy-post for GVHD prevention. Based on these promising results in terms of efficacy and safety, enrolment of new patients is currently ongoing.
Session topic: 36. Quality of life, palliative care, ethics and health economics


