
Contributions
Abstract: PB1809
Type: Publication Only
Background
Turoctocog alfa (NovoEight®, N8) is a third generation recombinant factor VIII, developed for prevention and treatment of bleeding episodes in patients with haemophilia A.
Aims
To investigate the efficacy and safety of turoctocog alfa as continuous infusion (CI) peri-operatively in patients with haemophilia A.
Methods
Ten adult patients undergoing eleven surgical procedures were included in this retrospective, observational cohort study. Primary objective was the efficacy of turoctocog alfa via CI based on the stability of FVIII levels, blood loss during surgery and bleeding complications. Secondary objective was the safety of turoctocog alfa as CI, also in terms of blood loss and bleeding complications and in terms of de novo FVIII-inhibitor development. Dose was started at 4 U/kg/hr and adjusted daily based on FVIII levels. Target levels were predefined.
Results
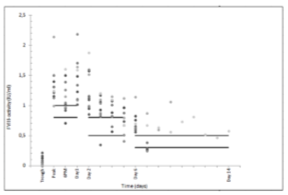
Ninety-three percent of the achieved FVIII levels were equivalent to or higher than the target levels, with a mean deviation of 37% above target. All patients showed a higher in vivo recovery than expected, with a mean recovery of 0,029 IU/kg. All patients had an adequate perioperative haemostatic response. A change in treatment because of a bleeding complication was not required in any case. There were no thromboembolic complications. No new cases of FVIII-inhibitors were detected.
Conclusion
Continuous infusion of turoctocog alfa is a feasible, effective and safe way of treating patients with haemophilia A during surgery.
Session topic: 34. Bleeding disorders (congenital and acquired)
Keyword(s): Surgery, Hemophilia A, Recombinant factor
Abstract: PB1809
Type: Publication Only
Background
Turoctocog alfa (NovoEight®, N8) is a third generation recombinant factor VIII, developed for prevention and treatment of bleeding episodes in patients with haemophilia A.
Aims
To investigate the efficacy and safety of turoctocog alfa as continuous infusion (CI) peri-operatively in patients with haemophilia A.
Methods
Ten adult patients undergoing eleven surgical procedures were included in this retrospective, observational cohort study. Primary objective was the efficacy of turoctocog alfa via CI based on the stability of FVIII levels, blood loss during surgery and bleeding complications. Secondary objective was the safety of turoctocog alfa as CI, also in terms of blood loss and bleeding complications and in terms of de novo FVIII-inhibitor development. Dose was started at 4 U/kg/hr and adjusted daily based on FVIII levels. Target levels were predefined.
Results
Ninety-three percent of the achieved FVIII levels were equivalent to or higher than the target levels, with a mean deviation of 37% above target. All patients showed a higher in vivo recovery than expected, with a mean recovery of 0,029 IU/kg. All patients had an adequate perioperative haemostatic response. A change in treatment because of a bleeding complication was not required in any case. There were no thromboembolic complications. No new cases of FVIII-inhibitors were detected.

Conclusion
Continuous infusion of turoctocog alfa is a feasible, effective and safe way of treating patients with haemophilia A during surgery.
Session topic: 34. Bleeding disorders (congenital and acquired)
Keyword(s): Surgery, Hemophilia A, Recombinant factor


