
Contributions
Abstract: PB2356
Type: Publication Only
Background
cITP is an acquired autoimmune disorder defined by low platelet counts for >12 months from diagnosis, resulting in an ongoing risk of significant bleeding (Rodeghiero et al. Blood 2009). Common therapy includes immunosuppression with corticosteroids; however, associated complications may be problematic, particularly for young children. The randomized, multicenter, placebo-controlled PETIT (Bussel et al. Lancet Haematol 2015) and PETIT2 (Grainger et al. Lancet 2015) trials in patients 1–17 years old demonstrated eltrombopag (EPAG), an oral thrombopoietin receptor agonist, as a therapeutic option for children with previously treated cITP. However, long-term data in pediatric patients are limited. Here we report outcomes from an open-label, Phase III extension study of pediatric patients who completed PETIT2 from four centers in Russia.
Aims
To evaluate the long-term safety and tolerability of EPAG in pediatric patients with previously treated cITP.
Methods
Patients aged ≥1–18 years previously enrolled in PETIT2 with clinical benefit from EPAG, were enrolled in the extension. Patients/guardians provided written informed consent. Screening was followed by a single-arm treatment period. Starting EPAG dose was based on the patient’s dose at the end of PETIT2, and adjusted according to platelet count and the investigator’s clinical judgement (max 75 mg/day) to maintain a safe hemostatic range (~50–200x109/L). Frequency and severity of adverse events (AEs) were recorded (graded using CTCAE version 4.03), and hematology and blood chemistry regularly monitored. Patients were considered to have completed the study and EPAG discontinued within 3 months once the patient reached 18 years of age or EPAG received local regulatory approval for pediatric cITP.
Results
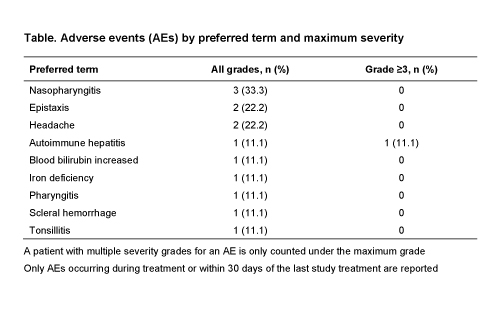
Of nine patients enrolled (4 female, 5 male; median 9 [range 4–15] years old), four (44.4%) completed the study and five (55.6%) discontinued (patient decision, n=2; lack of efficacy, n=2; AE [autoimmune hepatitis], n=1). Median duration of exposure in the extension was 24.6 (range 3–48) months; all nine patients received EPAG for ≥3 months. 8/9 patients started on or were escalated to 75 mg/day. AEs were reported in eight (88.9%) patients (Table), most commonly nasopharyngitis (n=3), epistaxis (n=2), and headache (n=2). No AE was considered EPAG related by the investigator. Serious AEs were reported in three (33.3%) patients: autoimmune hepatitis (n=1), epistaxis (n=1), and scleral hemorrhage (n=1); none were considered treatment related by the investigator. The autoimmune hepatitis case occurred in a 6 year old after ~3 months on EPAG, while on 75 mg/day; assessments at the time revealed Grade 3 alanine and aspartate aminotransferase (ALT/AST) elevations with normal bilirubin, ALT/AST normalized over the next 3 weeks following discontinuation.
Overall, platelet counts fluctuated, but were generally maintained within a safe hemostatic range; patient numbers per timepoint were low.
Conclusion
This open-label, Phase III extension study evaluated the long-term safety of EPAG in pediatric cITP patients who previously participated in PETIT2. Patients continued to receive benefit from EPAG with a safety profile consistent with that observed in the PETIT studies and the known safety profile for EPAG. Only one patient discontinued treatment because of an AE (autoimmune hepatitis), considered unrelated to EPAG by the investigator. There were no unexpected safety findings, indicating a favorable benefit–risk profile for EPAG in the long-term treatment of pediatric cITP patients.
Session topic: 33. Platelets disorders
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP), Thrombopoietin (TPO)
Abstract: PB2356
Type: Publication Only
Background
cITP is an acquired autoimmune disorder defined by low platelet counts for >12 months from diagnosis, resulting in an ongoing risk of significant bleeding (Rodeghiero et al. Blood 2009). Common therapy includes immunosuppression with corticosteroids; however, associated complications may be problematic, particularly for young children. The randomized, multicenter, placebo-controlled PETIT (Bussel et al. Lancet Haematol 2015) and PETIT2 (Grainger et al. Lancet 2015) trials in patients 1–17 years old demonstrated eltrombopag (EPAG), an oral thrombopoietin receptor agonist, as a therapeutic option for children with previously treated cITP. However, long-term data in pediatric patients are limited. Here we report outcomes from an open-label, Phase III extension study of pediatric patients who completed PETIT2 from four centers in Russia.
Aims
To evaluate the long-term safety and tolerability of EPAG in pediatric patients with previously treated cITP.
Methods
Patients aged ≥1–18 years previously enrolled in PETIT2 with clinical benefit from EPAG, were enrolled in the extension. Patients/guardians provided written informed consent. Screening was followed by a single-arm treatment period. Starting EPAG dose was based on the patient’s dose at the end of PETIT2, and adjusted according to platelet count and the investigator’s clinical judgement (max 75 mg/day) to maintain a safe hemostatic range (~50–200x109/L). Frequency and severity of adverse events (AEs) were recorded (graded using CTCAE version 4.03), and hematology and blood chemistry regularly monitored. Patients were considered to have completed the study and EPAG discontinued within 3 months once the patient reached 18 years of age or EPAG received local regulatory approval for pediatric cITP.
Results
Of nine patients enrolled (4 female, 5 male; median 9 [range 4–15] years old), four (44.4%) completed the study and five (55.6%) discontinued (patient decision, n=2; lack of efficacy, n=2; AE [autoimmune hepatitis], n=1). Median duration of exposure in the extension was 24.6 (range 3–48) months; all nine patients received EPAG for ≥3 months. 8/9 patients started on or were escalated to 75 mg/day. AEs were reported in eight (88.9%) patients (Table), most commonly nasopharyngitis (n=3), epistaxis (n=2), and headache (n=2). No AE was considered EPAG related by the investigator. Serious AEs were reported in three (33.3%) patients: autoimmune hepatitis (n=1), epistaxis (n=1), and scleral hemorrhage (n=1); none were considered treatment related by the investigator. The autoimmune hepatitis case occurred in a 6 year old after ~3 months on EPAG, while on 75 mg/day; assessments at the time revealed Grade 3 alanine and aspartate aminotransferase (ALT/AST) elevations with normal bilirubin, ALT/AST normalized over the next 3 weeks following discontinuation.
Overall, platelet counts fluctuated, but were generally maintained within a safe hemostatic range; patient numbers per timepoint were low.

Conclusion
This open-label, Phase III extension study evaluated the long-term safety of EPAG in pediatric cITP patients who previously participated in PETIT2. Patients continued to receive benefit from EPAG with a safety profile consistent with that observed in the PETIT studies and the known safety profile for EPAG. Only one patient discontinued treatment because of an AE (autoimmune hepatitis), considered unrelated to EPAG by the investigator. There were no unexpected safety findings, indicating a favorable benefit–risk profile for EPAG in the long-term treatment of pediatric cITP patients.
Session topic: 33. Platelets disorders
Keyword(s): Chronic ITP, Immune thrombocytopenia (ITP), Thrombopoietin (TPO)


