
Contributions
Abstract: PB2350
Type: Publication Only
Background
Patients with moderate-severe chronic thrombocytopenia have an increased risk of bleeding during surgical procedures. Perioperative management of these patients may be difficult. Eltrombopag is a well established treatment for chronic immune thrombocytopenia (ITP). During the last years, new indications for this agent have been proposed. Eltrombopag may be an interesting option for patients with different thrombocytopenic disorders requiring surgical interventions.
Aims
The aim of this study was to evaluate the efficacy and safety of perioperative use of Eltrombopag in thrombocytopenic patients undergoing invasive interventions.
Methods
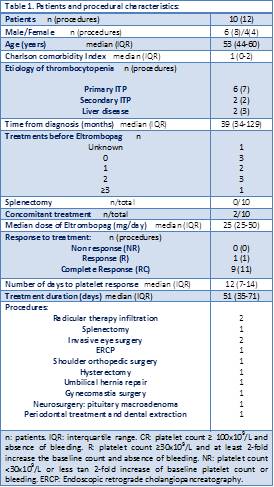
We retrospectively analyzed a total of 12 procedures carried out in 10 patients with chronic thrombocytopenia who received Eltrombopag. One patient was Jehovah’s Witness. We included patients with chronic ITP and thrombocytopenia due to liver disease. The data was obtained from the hospital computer records. Patients and procedure characteristics are shown at table 1. Eltrombopag was administered at a median initial dose of 25 mg/day (IQR, 25-50). To evaluate treatment efectiveness based on platelet response we used the International Working Group Consensus Criteria for ITP, defyning “Partial Response” and “Response” as platelet count ≥ 30x 109 or ≥100x109/L in absence of bleeding, respectively, and “Non response” as platelet count < 30x109/L or less than 2-fold increase of baseline platelet count or bleeding. Statistic analysis was performed using SPSS software, version 21.0.
Results
Median platelet count at initiation of Eltrombopag was 32 × 109 /L (IQR, 28 – 56 × 109 /L). All patients achieved response over a median of 12 days (IQR, 7-14). Median platelet count before intervention was 116 × 109 /L (IQR, 104-126 × 109 /L). There were no procedure cancellations due to thrombocytopenia. No patient needed rescue treatments or platelet transfusion during procedures. No several bleeding or thromboembolic event was recorded. No adverse effect was registered in our cohort. After surgical intervention, all patients discontinued Eltrombopag progressively with no rebound effect.
Conclusion
Eltrombopag may be an effective perioperative treatment for patients with thrombocytopenia of a variety of etiologies. This agent increases platelet count in a short period of time, what may reduce the risk of bleeding of patients undergoing elective invasive interventions. It is a good alternative for patients with ITP who are not candidates to classical therapies as corticoids or immunoglobulins and its use may avoid perioperative platelet transfusion. Treatment with Eltrombopag may be discontinued safely after procedures in patients not requiring chronic treatment. Properly designed clinical trials are needed to verify these findings.
Session topic: 33. Platelets disorders
Keyword(s): Immune thrombocytopenia (ITP), Platelet transfusion, Surgery, Thrombopoietin (TPO)
Abstract: PB2350
Type: Publication Only
Background
Patients with moderate-severe chronic thrombocytopenia have an increased risk of bleeding during surgical procedures. Perioperative management of these patients may be difficult. Eltrombopag is a well established treatment for chronic immune thrombocytopenia (ITP). During the last years, new indications for this agent have been proposed. Eltrombopag may be an interesting option for patients with different thrombocytopenic disorders requiring surgical interventions.
Aims
The aim of this study was to evaluate the efficacy and safety of perioperative use of Eltrombopag in thrombocytopenic patients undergoing invasive interventions.
Methods
We retrospectively analyzed a total of 12 procedures carried out in 10 patients with chronic thrombocytopenia who received Eltrombopag. One patient was Jehovah’s Witness. We included patients with chronic ITP and thrombocytopenia due to liver disease. The data was obtained from the hospital computer records. Patients and procedure characteristics are shown at table 1. Eltrombopag was administered at a median initial dose of 25 mg/day (IQR, 25-50). To evaluate treatment efectiveness based on platelet response we used the International Working Group Consensus Criteria for ITP, defyning “Partial Response” and “Response” as platelet count ≥ 30x 109 or ≥100x109/L in absence of bleeding, respectively, and “Non response” as platelet count < 30x109/L or less than 2-fold increase of baseline platelet count or bleeding. Statistic analysis was performed using SPSS software, version 21.0.
Results
Median platelet count at initiation of Eltrombopag was 32 × 109 /L (IQR, 28 – 56 × 109 /L). All patients achieved response over a median of 12 days (IQR, 7-14). Median platelet count before intervention was 116 × 109 /L (IQR, 104-126 × 109 /L). There were no procedure cancellations due to thrombocytopenia. No patient needed rescue treatments or platelet transfusion during procedures. No several bleeding or thromboembolic event was recorded. No adverse effect was registered in our cohort. After surgical intervention, all patients discontinued Eltrombopag progressively with no rebound effect.

Conclusion
Eltrombopag may be an effective perioperative treatment for patients with thrombocytopenia of a variety of etiologies. This agent increases platelet count in a short period of time, what may reduce the risk of bleeding of patients undergoing elective invasive interventions. It is a good alternative for patients with ITP who are not candidates to classical therapies as corticoids or immunoglobulins and its use may avoid perioperative platelet transfusion. Treatment with Eltrombopag may be discontinued safely after procedures in patients not requiring chronic treatment. Properly designed clinical trials are needed to verify these findings.
Session topic: 33. Platelets disorders
Keyword(s): Immune thrombocytopenia (ITP), Platelet transfusion, Surgery, Thrombopoietin (TPO)


