
Contributions
Abstract: PB2424
Type: Publication Only
Background
The E2A-PBX1 rearrangement is common in B-cell acute lymphoblastic leukemia (B-ALL). However, whether this fusion gene can be used as a reliable marker for minimal residual disease (MRD) following allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains unknown.
Aims
The aim of this study was to investigate the clinical characteristics of E2A-PBX1(immunoglobulin
enhancer binding factor-pre-B leukemia) fusion gene in patients with acute lymphoblastic leukemia (ALL) after allogeneic stem cell transplantation (allo- HSCT).
Methods
Clinical data were collected from 28 consecutive B-ALL patients who received allo-HSCT. The E2A-PBX1 gene was examined by real-time quantitative polymerase chain reaction (RQ- PCR). The correlation between its expression level and the disease status was analyzed.
Results
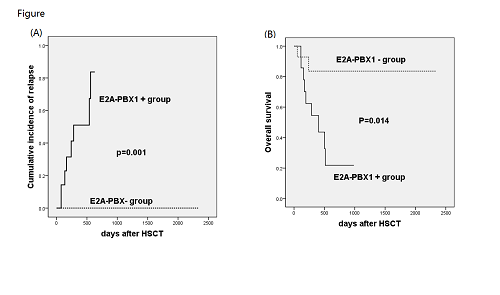
The median follow-up was 374d (55-2342d). Of the enrolled patients, 7 (25%) patients died of leukemia relapse. A total of 9 (32.1%) patients experienced relapse at a median of 164d (75-559d) after transplantation. The median expression level in the first positive sample was 0.14% (0.0071-902.4%). The duration from E2A-PBX1-positive results to hematological relapse was 74d (30-469d). E2A-PBX1 expression generally became positive prior to flow cytometry. Patients with positive E2A-PBX1 gene expression pre-transplantation were more likely to have positive E2A-PBX1 expression after transplantation.
Conclusion
E2A-PBX1 expression determined by real-time quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) could be used to evaluate MRD status after allo-HSCT. Patients with positive E2A-PBX1 expression after transplant will have a poor prognosis.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Acute lymphoblastic leukemia, Minimal residual disease (MRD), Real time PCR, Stem cell transplant
Abstract: PB2424
Type: Publication Only
Background
The E2A-PBX1 rearrangement is common in B-cell acute lymphoblastic leukemia (B-ALL). However, whether this fusion gene can be used as a reliable marker for minimal residual disease (MRD) following allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains unknown.
Aims
The aim of this study was to investigate the clinical characteristics of E2A-PBX1(immunoglobulin
enhancer binding factor-pre-B leukemia) fusion gene in patients with acute lymphoblastic leukemia (ALL) after allogeneic stem cell transplantation (allo- HSCT).
Methods
Clinical data were collected from 28 consecutive B-ALL patients who received allo-HSCT. The E2A-PBX1 gene was examined by real-time quantitative polymerase chain reaction (RQ- PCR). The correlation between its expression level and the disease status was analyzed.
Results
The median follow-up was 374d (55-2342d). Of the enrolled patients, 7 (25%) patients died of leukemia relapse. A total of 9 (32.1%) patients experienced relapse at a median of 164d (75-559d) after transplantation. The median expression level in the first positive sample was 0.14% (0.0071-902.4%). The duration from E2A-PBX1-positive results to hematological relapse was 74d (30-469d). E2A-PBX1 expression generally became positive prior to flow cytometry. Patients with positive E2A-PBX1 gene expression pre-transplantation were more likely to have positive E2A-PBX1 expression after transplantation.

Conclusion
E2A-PBX1 expression determined by real-time quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) could be used to evaluate MRD status after allo-HSCT. Patients with positive E2A-PBX1 expression after transplant will have a poor prognosis.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Acute lymphoblastic leukemia, Minimal residual disease (MRD), Real time PCR, Stem cell transplant


