
Contributions
Abstract: PB2476
Type: Publication Only
Background
We analyzed 24 symptomatic newly diagnosed Multiple Myeloma (NDMM) patients who were 65 years or older receiving induction therapy with novel agents plus ASCT.
Aims
The objectives were to assess the toxicity and efficacy in two cohorts: elderly patients (≥70 years or older) versus a younger group (65-69 years).
Methods
The endpoints were: overall response rate (ORR), progression free survival (PFS) and overall survival (OS), adverse effects, time to platelet and neutrophils engraftment and time to discharge. The dose of melphalan conditioning employed were 200 or reduced doses (140, 100 or 70 mg/m2 in tandem) if age ≥70 years, renal failure or Hematopoietic Cell Transplantation-Comorbidity Index over 2 were presented at ASCT moment.
Results
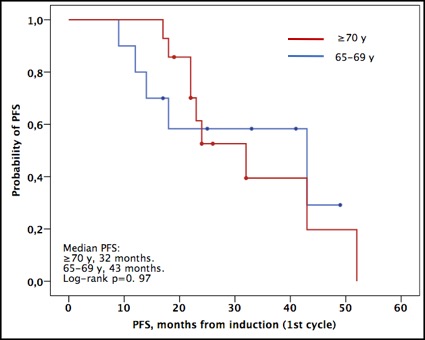
14 patients ≥70 y and 10 patients between 65-69 years were reported. ORR an OS rate at 1 year was 100%. No differences were observed in terms of time to reach platelets and neutrophils engraphtments, time to discharge and adverse effects. After a median follow-up of 23.5 months, the median PFS was 32 months in the older group, as compared with 43 months in the group down 70 years underwent ASCT (p=0.97).
Conclusion
ASCT is feasible and safe in NDMM patients aged ≥70 years if an individualized approach is performed.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Myeloma, Complete Remission, Elderly, Melphalan
Abstract: PB2476
Type: Publication Only
Background
We analyzed 24 symptomatic newly diagnosed Multiple Myeloma (NDMM) patients who were 65 years or older receiving induction therapy with novel agents plus ASCT.
Aims
The objectives were to assess the toxicity and efficacy in two cohorts: elderly patients (≥70 years or older) versus a younger group (65-69 years).
Methods
The endpoints were: overall response rate (ORR), progression free survival (PFS) and overall survival (OS), adverse effects, time to platelet and neutrophils engraftment and time to discharge. The dose of melphalan conditioning employed were 200 or reduced doses (140, 100 or 70 mg/m2 in tandem) if age ≥70 years, renal failure or Hematopoietic Cell Transplantation-Comorbidity Index over 2 were presented at ASCT moment.
Results
14 patients ≥70 y and 10 patients between 65-69 years were reported. ORR an OS rate at 1 year was 100%. No differences were observed in terms of time to reach platelets and neutrophils engraphtments, time to discharge and adverse effects. After a median follow-up of 23.5 months, the median PFS was 32 months in the older group, as compared with 43 months in the group down 70 years underwent ASCT (p=0.97).

Conclusion
ASCT is feasible and safe in NDMM patients aged ≥70 years if an individualized approach is performed.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Myeloma, Complete Remission, Elderly, Melphalan


