
Contributions
Abstract: PB2417
Type: Publication Only
Background
An important point for success of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a sufficient and durable reconstitution of hematopoietic donor stem cells. Primary poor graft function (PPGF) after allo-HSCT is characterized by persistent pancytopenia, immunodeficiency and dependence on blood transfusions, despite of a complete donor chimerism. Absence of a regulatory effect of bone marrow stromal microenvironment in patients with aplastic anemia, even in case of using bone marrow as a source of stem cells, increases the probability of development of PPGF.
Aims
The purpose of this analysis is to assess safety, efficacy and survival after CD34+-selected stem cell boost in patients (pts) with PPGF after allo-HSCT for aplastic anemia.
Methods
The analysis comprises data of 13 pts with aplastic anemia after allo-HSCT from HLA-matched sibling (n=7) or unrelated (n= 6) donors, who underwent allo-HSCT between 2008 and 2017 . The median age of the 10 male and 3 female pts was 32 years (range, 17 to 66). The immunoablative conditioning regimen consisting of 4 x 50 mg/kg cyclophosphamide and 3 x 30 mg/kg rabbit ATG was performed in all pts before allo-HSCT. In case of unrelated donors, 2 Gy total nodal irradiation was additionaly administerd. Bone marrow (n=12) or peripheral blood stem cells (n=1) with the a median of 2.65 x 106 CD34+ cells/kg bodyweight (BW)(range, 1.5 to 4.7) were transplanted. PPGF was defined as transfusion-dependent thrombocytopenia with platelet counts < 20.000/µl, or transfusion-dependent anemia or leucopenia with neutrophil counts < 1.000/µl. PPGF was diagnosed at a median of 47 days (range, 22 to 100) after allo-HSCT by bone marrow biopsy. The median neutrophil count at time of stem cell boost administration was 0.3 x 109/L (range, 0.14 to 1.8), the median platelet count 15 x 109/L (range, 7 to 30), and the median hemoglobin concentration 8 g/dL (range, 7 to 9), respectively. Immunomagnetically selected CD34+ stem cells from original donors were used without further immunosuppressive prophylaxis. Cell counts were as follows: 5.48 x 106 CD34+ cells/kg BW (range, 0.95 to 13.2), 0.22 x 104 CD3+ cells/kg BW (range, 0.16 to 0.8), 2,12 x 104 CD20+ cells/kg BW (range, 0.55 to 3.55). Hematological engraftment was defined as an increase of neutrophils by 1.000/µl, of platelets by 50.000/µl, and hemoglobin by 8.5g/dL.
Results
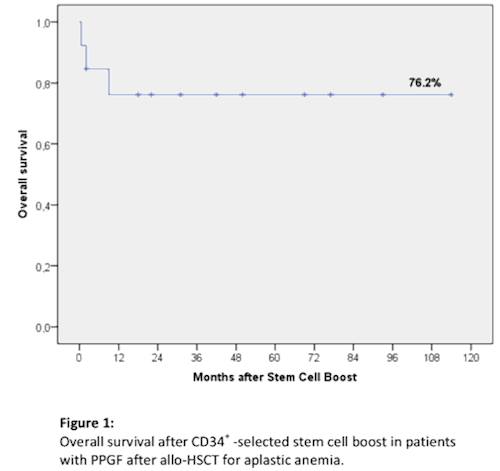
The rate of complete hematological engraftment was 85% (11 of 13 pts). A partial response in 1 or 2 hematopoietic cell lines was noted in 2 pts (15%). The median time to hematological engraftment was 17 days after stem cells boost. The current actuarial 9-year survival rate is 76% (Fig.1). In only one case, 8 months after stem cell boost, a relapse of aplastic anemia developed, requiring a second allo-HSCT. Three pts died as a result of infectious complications. Acute graft-versus-host disease was diagnosed in 3 pts (23%): grade I (skin, liver) and chronic GvHD in 5 pts (38%) (limited n = 4, extensive n =1).
Conclusion
The use of CD34+-selected peripheral blood stem cell boosts from the original donor allows a rapid regeneration of hematopoiesis in the majority of aplastic anemia patients with PPGF after allo-HSCT. Infectious complications might be decreased by deciding on early stem cell boost and improved anti-infectious prophylaxis in pts with PPGF.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Allogeneic hematopoietic stem cell transplant, Aplastic anemia, CD34+ cells, Engraftment
Abstract: PB2417
Type: Publication Only
Background
An important point for success of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a sufficient and durable reconstitution of hematopoietic donor stem cells. Primary poor graft function (PPGF) after allo-HSCT is characterized by persistent pancytopenia, immunodeficiency and dependence on blood transfusions, despite of a complete donor chimerism. Absence of a regulatory effect of bone marrow stromal microenvironment in patients with aplastic anemia, even in case of using bone marrow as a source of stem cells, increases the probability of development of PPGF.
Aims
The purpose of this analysis is to assess safety, efficacy and survival after CD34+-selected stem cell boost in patients (pts) with PPGF after allo-HSCT for aplastic anemia.
Methods
The analysis comprises data of 13 pts with aplastic anemia after allo-HSCT from HLA-matched sibling (n=7) or unrelated (n= 6) donors, who underwent allo-HSCT between 2008 and 2017 . The median age of the 10 male and 3 female pts was 32 years (range, 17 to 66). The immunoablative conditioning regimen consisting of 4 x 50 mg/kg cyclophosphamide and 3 x 30 mg/kg rabbit ATG was performed in all pts before allo-HSCT. In case of unrelated donors, 2 Gy total nodal irradiation was additionaly administerd. Bone marrow (n=12) or peripheral blood stem cells (n=1) with the a median of 2.65 x 106 CD34+ cells/kg bodyweight (BW)(range, 1.5 to 4.7) were transplanted. PPGF was defined as transfusion-dependent thrombocytopenia with platelet counts < 20.000/µl, or transfusion-dependent anemia or leucopenia with neutrophil counts < 1.000/µl. PPGF was diagnosed at a median of 47 days (range, 22 to 100) after allo-HSCT by bone marrow biopsy. The median neutrophil count at time of stem cell boost administration was 0.3 x 109/L (range, 0.14 to 1.8), the median platelet count 15 x 109/L (range, 7 to 30), and the median hemoglobin concentration 8 g/dL (range, 7 to 9), respectively. Immunomagnetically selected CD34+ stem cells from original donors were used without further immunosuppressive prophylaxis. Cell counts were as follows: 5.48 x 106 CD34+ cells/kg BW (range, 0.95 to 13.2), 0.22 x 104 CD3+ cells/kg BW (range, 0.16 to 0.8), 2,12 x 104 CD20+ cells/kg BW (range, 0.55 to 3.55). Hematological engraftment was defined as an increase of neutrophils by 1.000/µl, of platelets by 50.000/µl, and hemoglobin by 8.5g/dL.
Results
The rate of complete hematological engraftment was 85% (11 of 13 pts). A partial response in 1 or 2 hematopoietic cell lines was noted in 2 pts (15%). The median time to hematological engraftment was 17 days after stem cells boost. The current actuarial 9-year survival rate is 76% (Fig.1). In only one case, 8 months after stem cell boost, a relapse of aplastic anemia developed, requiring a second allo-HSCT. Three pts died as a result of infectious complications. Acute graft-versus-host disease was diagnosed in 3 pts (23%): grade I (skin, liver) and chronic GvHD in 5 pts (38%) (limited n = 4, extensive n =1).

Conclusion
The use of CD34+-selected peripheral blood stem cell boosts from the original donor allows a rapid regeneration of hematopoiesis in the majority of aplastic anemia patients with PPGF after allo-HSCT. Infectious complications might be decreased by deciding on early stem cell boost and improved anti-infectious prophylaxis in pts with PPGF.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Allogeneic hematopoietic stem cell transplant, Aplastic anemia, CD34+ cells, Engraftment


