
Contributions
Abstract: PB1760
Type: Publication Only
Background
Cisplatin containing regimens showed promising results as salvage chemotherapy (CT) in relapsed or refractory (R/R) lymphomas, mainly as bridge to autologous stem cells transplant (ASCT). Tolerability is mainly impaired by mucositis and cisplatin-associated renal and neurological toxicity. Oxaliplatin shows a better toxicity profile, but at the present time few data are available in R/R lymphomas setting.
Aims
To investigate the feasibility and efficacy of oxaliplatin, cytarabine and dexamethasone (DHAOx) as salvage and mobilizing regimen for peripheral blood stem cells (PBSC).
Methods
We retrospectively analyzed 83 R/R lymphoma patients diagnosed between October 2004 and October 2014, who received DHAOx as salvage CT. Forty-one had Diffuse Large B-cell Lymphoma (DLBCL, 49%), 10 Hodgkin Lymphoma (HL, 12%), 13 Mantle Cell Lymphoma (15%), 15 other Indolent Lymphomas (18%) and 4 Peripheral T-cell Lymphoma (5%). Median age was 59 (range 22-79). DHAOx schedule consists inq21 days-administration of dexamethasone 40 mg/die on days 1-4, oxaliplatin 130 mg/m2 on day 1, cytarabine 2 g/m2 bid on day 2.In B-cells lymphomas, Rituximab 375 mg/m2was added on day 2. G-CSF was administered from day 7 until absolute neutrophil count recovery or from day 5 to 10 (5 mcg/Kg/die) if PBSC collection was scheduled. We analyzed overall response rate(ORR), progression free survival (PFS), hematological and non-hematological toxicities and success rate of BPSC collection.
Results
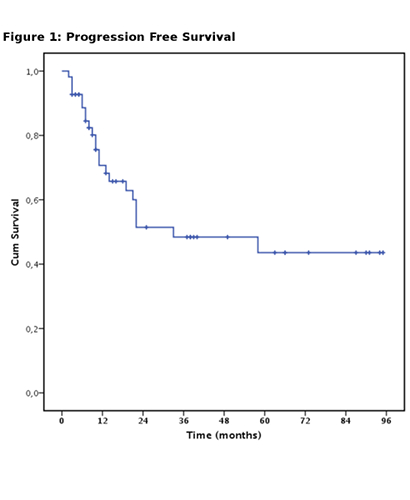
ORR was 64%, Complete remission (CR) was achieved in 48 patients (55%), partial remission (PR) in 8 patients (9%), 32 patients did not respond (36%). Patients affected by DLBCL had significantly higher probability of achieving CR if compared to HL, MCL and indolent lymphomas (p<0.05). Median PFS was 33 months (fig.1). The projected OS at 36 months was 54.5%. %. Survival was better in patients with DLBCL and HL (p<0.05).
Grade >2 non hematological toxicity was observed in 27 patients (33%),most frequently oral mucositis and diarrhea (6 cases, 8%), FUO (17 cases, 21%), sepsis (3 cases, 4%), paresthesia (5 patients, 6%). No patients experienced acute renal impairment.
Among DLBCL patients, 81% were treated in second line,19% in third or subsequent line. ORR was 71%. CR was obtained in 60%, PR in 11%. Eleven patients did not respond 29%. The median PFS was not reached and the projected OS at 36 months was 45.5.
Following DHAOx and G-CSF priming, stem cell harvest for ASCT was planned in 40 patients (48%), all of them successfully performed the procedure and reached the target of 4x10^6 CD34+/Kg. Only 1 heavily pretreated patient needed plerixafor administration.
All patients performing stem cell collection were able to proceed to ASCT.
Conclusion
Our experience shows that the use of oxaliplatin instead of cisplatin is feasible, alongside showing similar efficacy compared to conventional cisplatin containing regimens for R/R lymphomas. Low hematological and non-hematological toxicity as well as good capability of PBSC mobilization were observed. Notably, no renal toxicity was observed. The very good toxicity profile allowed all eligible responding patients to proceed with planned ASCT consolidation.
Oxaliplatin - high dose cytarabine salvage therapy is therefore a reasonable option, expecially for R/R DLBCL patients considered eligible for ASCT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Mobilization, NHL, Oxaliplatin, Refractory
Abstract: PB1760
Type: Publication Only
Background
Cisplatin containing regimens showed promising results as salvage chemotherapy (CT) in relapsed or refractory (R/R) lymphomas, mainly as bridge to autologous stem cells transplant (ASCT). Tolerability is mainly impaired by mucositis and cisplatin-associated renal and neurological toxicity. Oxaliplatin shows a better toxicity profile, but at the present time few data are available in R/R lymphomas setting.
Aims
To investigate the feasibility and efficacy of oxaliplatin, cytarabine and dexamethasone (DHAOx) as salvage and mobilizing regimen for peripheral blood stem cells (PBSC).
Methods
We retrospectively analyzed 83 R/R lymphoma patients diagnosed between October 2004 and October 2014, who received DHAOx as salvage CT. Forty-one had Diffuse Large B-cell Lymphoma (DLBCL, 49%), 10 Hodgkin Lymphoma (HL, 12%), 13 Mantle Cell Lymphoma (15%), 15 other Indolent Lymphomas (18%) and 4 Peripheral T-cell Lymphoma (5%). Median age was 59 (range 22-79). DHAOx schedule consists inq21 days-administration of dexamethasone 40 mg/die on days 1-4, oxaliplatin 130 mg/m2 on day 1, cytarabine 2 g/m2 bid on day 2.In B-cells lymphomas, Rituximab 375 mg/m2was added on day 2. G-CSF was administered from day 7 until absolute neutrophil count recovery or from day 5 to 10 (5 mcg/Kg/die) if PBSC collection was scheduled. We analyzed overall response rate(ORR), progression free survival (PFS), hematological and non-hematological toxicities and success rate of BPSC collection.
Results
ORR was 64%, Complete remission (CR) was achieved in 48 patients (55%), partial remission (PR) in 8 patients (9%), 32 patients did not respond (36%). Patients affected by DLBCL had significantly higher probability of achieving CR if compared to HL, MCL and indolent lymphomas (p<0.05). Median PFS was 33 months (fig.1). The projected OS at 36 months was 54.5%. %. Survival was better in patients with DLBCL and HL (p<0.05).
Grade >2 non hematological toxicity was observed in 27 patients (33%),most frequently oral mucositis and diarrhea (6 cases, 8%), FUO (17 cases, 21%), sepsis (3 cases, 4%), paresthesia (5 patients, 6%). No patients experienced acute renal impairment.
Among DLBCL patients, 81% were treated in second line,19% in third or subsequent line. ORR was 71%. CR was obtained in 60%, PR in 11%. Eleven patients did not respond 29%. The median PFS was not reached and the projected OS at 36 months was 45.5.
Following DHAOx and G-CSF priming, stem cell harvest for ASCT was planned in 40 patients (48%), all of them successfully performed the procedure and reached the target of 4x10^6 CD34+/Kg. Only 1 heavily pretreated patient needed plerixafor administration.
All patients performing stem cell collection were able to proceed to ASCT.

Conclusion
Our experience shows that the use of oxaliplatin instead of cisplatin is feasible, alongside showing similar efficacy compared to conventional cisplatin containing regimens for R/R lymphomas. Low hematological and non-hematological toxicity as well as good capability of PBSC mobilization were observed. Notably, no renal toxicity was observed. The very good toxicity profile allowed all eligible responding patients to proceed with planned ASCT consolidation.
Oxaliplatin - high dose cytarabine salvage therapy is therefore a reasonable option, expecially for R/R DLBCL patients considered eligible for ASCT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Mobilization, NHL, Oxaliplatin, Refractory


