
Contributions
Abstract: PB1763
Type: Publication Only
Background
There is great unmet need in improving the prognosis of patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL).
Aims
How to get those patients into remission and enable them to receive autologous stem cell transplantation (ASCT) is a great challenge.
Methods
A phase 2 clinical trial was conducted to evaluate the efficacy and safety of rituximab in combination with two nanoparticle-delivered chemotherapy drugs, albumin-bound paclitaxel and pegylated liposomal doxorubicin (RAD) in patients with relapsed/refractory DLBCL who received at least two prior lines of immunochemotherapy.
Results
13 patients were enrolled with a median age of 40 years. All patients received a median of 3 prior lines of therapy. 12 patients received at least two cycles of RAD. After 2 cycles of RAD, the complete response rate was 38.5%, partial response rate was 46.2%, and overall response rate was 84.6%. Three patients successfully received subsequent ASCT, and were still alive without disease after a follow-up time of 4 months, 15 months, and 29 months, respectively. Three patients died of disease progression when waiting for ASCT, and another three patients failed collection of stem cells due to bone marrow dysfunction. This RAD regimen was well tolerated with mostly reported adverse events being grade 2 or 3 hematologic events.
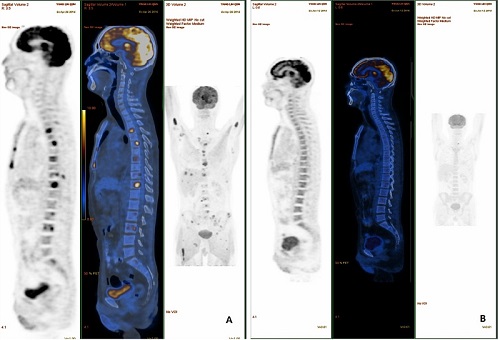
Conclusion
In conclusion, we demonstrated that rituximab in combination with albumin-bound paclitaxel and pegylated liposomal doxorubicin was highly active in patients with relapsed/refractory DLBCL, and this regimen should be considered as an effective salvage therapy to bridge subsequent ASCT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, Relapsed lymphoma, Salvage chemotherapy
Abstract: PB1763
Type: Publication Only
Background
There is great unmet need in improving the prognosis of patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL).
Aims
How to get those patients into remission and enable them to receive autologous stem cell transplantation (ASCT) is a great challenge.
Methods
A phase 2 clinical trial was conducted to evaluate the efficacy and safety of rituximab in combination with two nanoparticle-delivered chemotherapy drugs, albumin-bound paclitaxel and pegylated liposomal doxorubicin (RAD) in patients with relapsed/refractory DLBCL who received at least two prior lines of immunochemotherapy.
Results
13 patients were enrolled with a median age of 40 years. All patients received a median of 3 prior lines of therapy. 12 patients received at least two cycles of RAD. After 2 cycles of RAD, the complete response rate was 38.5%, partial response rate was 46.2%, and overall response rate was 84.6%. Three patients successfully received subsequent ASCT, and were still alive without disease after a follow-up time of 4 months, 15 months, and 29 months, respectively. Three patients died of disease progression when waiting for ASCT, and another three patients failed collection of stem cells due to bone marrow dysfunction. This RAD regimen was well tolerated with mostly reported adverse events being grade 2 or 3 hematologic events.

Conclusion
In conclusion, we demonstrated that rituximab in combination with albumin-bound paclitaxel and pegylated liposomal doxorubicin was highly active in patients with relapsed/refractory DLBCL, and this regimen should be considered as an effective salvage therapy to bridge subsequent ASCT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, Relapsed lymphoma, Salvage chemotherapy


