
Contributions
Abstract: PB1781
Type: Publication Only
Background
Sézary syndrome (SS) is a rare (7.7 cases per million per year) and incurable disease, it is one of the major variants of cutaneous T-cell lymphomas (CTCL). Extracorporeal photopheresis (ECP) is a process by which peripheral blood mononuclear cells are isolated from circulation by discontinuous leukapheresis, exposed ex-vivo to pro-apoptotic doses of 8-methoxypsoralen and ultraviolet A radiation and reinfused. Nowadays is a technique used for different lymphocyte-mediated diseases, including CTCL, graft-versus-host disease and solid organ transplant rejection.
Aims
To analyze response rate and duration of response of ECP for advanced stage MF/SS.
Methods
Patients >18y.o, diagnosed with SS not previously transplanted treated and treated with ECP were consecutively included since August 2015. The ECP protocol included 2 procedures administered on 2 consecutive days at 2-weeks intervals until the procedure number 20, when an evaluation was performed (ECP20) assessing the skin (dermatological) and hematological responses according to Olsen criteria (Olsen et al, JCO 2011;29(18):2598-607). For complete dermatological response (CDR): 100% skin clearance, partial DR (PDR): 50-99% clearance of lesions without new tumors, stable D disease (SDD): <25% increase or <50% clearance of skin lesions, progressive D disease (PrDD) >25% increase or new tumors, for blood involvement: complete hematological response (CHR): B0, partial HR (PHR): >50% decrease in Sézary cells, progressive H disease (PrHD): move form B0 to B2 or >50% increase in blood burden with at elast 5,000 Sézary cells/microL, stable HD (SHD): fails to CHR, PHR or PrHD. In cases without response or progressive disease (PD), ECP was stopped; in cases with partial response (PR), bi-weekly ECP is continued until procedure number 30, in cases with complete response (CR), ECP continued 2 consecutive days every 4-week with progressive withdrawal in 10 procedures.
Results
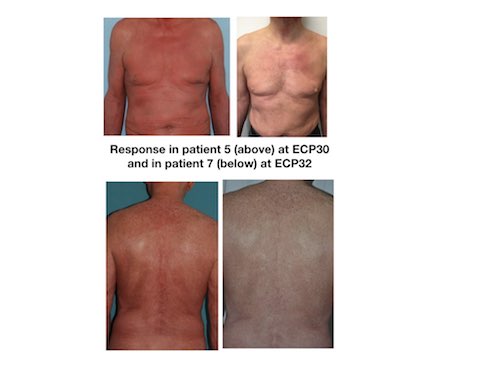
9 patients were eligible for ECP, 7 completed at least 20 procedures and were included in the analysis, 1 patient discontinued therapy due to PD after 18 ECP, and 1 patient due to a new diagnosis of a degenerative disease (lateral amyotrophic sclerosis). Among the 7 patients that could be evaluated at ECP20, 4 were males, mean age was 62 y.o (range: 36-72), and stage was IVA1in 5 and IVA2 in 2. In 4 patients, ECP was started in progression at a median time from diagnosis of 10 months (limits 1-101) (median previous lines 3), and in 3 cases was used as front-line therapy combined with prednisone, retinoids or interferon. ECP20 evaluation showed CR in 1 patient and PR in 6. Specific 20ECP responses were: PHR+SDD: 2, PHR+PDR: 2, CHR+PDR: 2, CHR+CDR: 1. After ECP20, 3 patients (1CHR+CDR, 2 CHR+PDR) continued bi-weekly ECP until procedure 30 and then were switched to the 4-week schedule, the other 4 patients continued in the 2-weeks schedule. With a median follow-up of 19 months (limits 8-25), none of the 7 patients lost their response. Median duration of response (PFS since ECP20) was 14 (limits 23-21) months. Currently 1 patient continues in CHR+CDR, 4 are in CHR+PDR and 2 in PHR+PDR. The patient in CHR+CDR stopped ECP after 34 procedures and continued free of relapse 21 months later. No mortalities neither hospitalization were registered during therapy. Fig1 shows two illustrative cases.
Conclusion
Patients showed long-lasting responses with ECP without toxicity. Moreover, none of the responder patients at ECP20 lost their response during the follow-up.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Cutaneous T-cell lymphoma, Photopheresis
Abstract: PB1781
Type: Publication Only
Background
Sézary syndrome (SS) is a rare (7.7 cases per million per year) and incurable disease, it is one of the major variants of cutaneous T-cell lymphomas (CTCL). Extracorporeal photopheresis (ECP) is a process by which peripheral blood mononuclear cells are isolated from circulation by discontinuous leukapheresis, exposed ex-vivo to pro-apoptotic doses of 8-methoxypsoralen and ultraviolet A radiation and reinfused. Nowadays is a technique used for different lymphocyte-mediated diseases, including CTCL, graft-versus-host disease and solid organ transplant rejection.
Aims
To analyze response rate and duration of response of ECP for advanced stage MF/SS.
Methods
Patients >18y.o, diagnosed with SS not previously transplanted treated and treated with ECP were consecutively included since August 2015. The ECP protocol included 2 procedures administered on 2 consecutive days at 2-weeks intervals until the procedure number 20, when an evaluation was performed (ECP20) assessing the skin (dermatological) and hematological responses according to Olsen criteria (Olsen et al, JCO 2011;29(18):2598-607). For complete dermatological response (CDR): 100% skin clearance, partial DR (PDR): 50-99% clearance of lesions without new tumors, stable D disease (SDD): <25% increase or <50% clearance of skin lesions, progressive D disease (PrDD) >25% increase or new tumors, for blood involvement: complete hematological response (CHR): B0, partial HR (PHR): >50% decrease in Sézary cells, progressive H disease (PrHD): move form B0 to B2 or >50% increase in blood burden with at elast 5,000 Sézary cells/microL, stable HD (SHD): fails to CHR, PHR or PrHD. In cases without response or progressive disease (PD), ECP was stopped; in cases with partial response (PR), bi-weekly ECP is continued until procedure number 30, in cases with complete response (CR), ECP continued 2 consecutive days every 4-week with progressive withdrawal in 10 procedures.
Results
9 patients were eligible for ECP, 7 completed at least 20 procedures and were included in the analysis, 1 patient discontinued therapy due to PD after 18 ECP, and 1 patient due to a new diagnosis of a degenerative disease (lateral amyotrophic sclerosis). Among the 7 patients that could be evaluated at ECP20, 4 were males, mean age was 62 y.o (range: 36-72), and stage was IVA1in 5 and IVA2 in 2. In 4 patients, ECP was started in progression at a median time from diagnosis of 10 months (limits 1-101) (median previous lines 3), and in 3 cases was used as front-line therapy combined with prednisone, retinoids or interferon. ECP20 evaluation showed CR in 1 patient and PR in 6. Specific 20ECP responses were: PHR+SDD: 2, PHR+PDR: 2, CHR+PDR: 2, CHR+CDR: 1. After ECP20, 3 patients (1CHR+CDR, 2 CHR+PDR) continued bi-weekly ECP until procedure 30 and then were switched to the 4-week schedule, the other 4 patients continued in the 2-weeks schedule. With a median follow-up of 19 months (limits 8-25), none of the 7 patients lost their response. Median duration of response (PFS since ECP20) was 14 (limits 23-21) months. Currently 1 patient continues in CHR+CDR, 4 are in CHR+PDR and 2 in PHR+PDR. The patient in CHR+CDR stopped ECP after 34 procedures and continued free of relapse 21 months later. No mortalities neither hospitalization were registered during therapy. Fig1 shows two illustrative cases.

Conclusion
Patients showed long-lasting responses with ECP without toxicity. Moreover, none of the responder patients at ECP20 lost their response during the follow-up.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Cutaneous T-cell lymphoma, Photopheresis


