
Contributions
Abstract: PB2325
Type: Publication Only
Background
Mycosis fungoides (MF) is the most frequent cutaneous T-cell lymphoma, generally with indolent course. It is accepted as a marker of poor prognosis a tumor load in peripheral blood of more than 1,000 cells/microL or more than 20% of the lymphocytes (B2), and equivalent to the nodal involvement (1). Less evidence exists on whether the detection of a lower tumor load in peripheral blood (B1<1,000 cel/microL) may have prognostic implications. In these cases of low tumor load, the manual Sézary count is difficult and requires a high experience. Techniques such as flow cytometry (FCM) and PCR (TCR gamma rearrangement) can help us to detect the presence of peripheral blood disease. Recently, the EORTC lymphoma cutaneous task force has recommended the FCM definition of blood-class even in patch/plaque/tumor MF (2), although the clinical implication of this minor blood involvement is to be defined.
Aims
To assess the degree of agreement between the results in the detection of Sézary cells in peripheral blood between FCM and PCR and the prognostic significance of the detection of low tumor load (stage B1).
Methods
The results obtained from 49 patients diagnosed of MF were retrospectively studied. The presence of circulating Sézary cells was analyzed by two techniques. For FCM, according to EORTC recommendations (1), presence of circulating Sézary cells was considered when the CD4/CD8 ratio was ≥10, or when the percentage of CD4 T negative for CD7>40% and/or for CD26>30%. The TCR gamma rearrangement was performed in parallel to identify clonal T cells. In addition, staging and survival data were collected.
Results
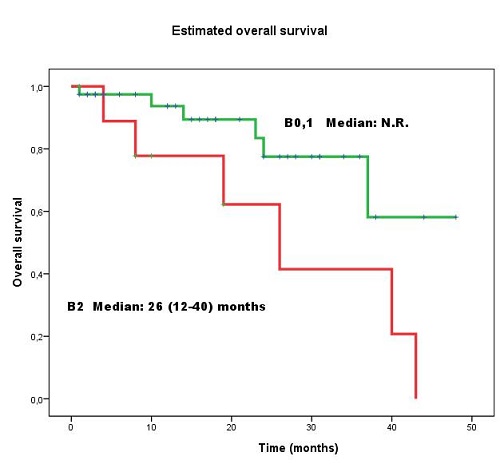
We identified 49 patients with MF, a median age 62 (19-88) years and 60% males. Samples of peripheral blood were sent in parallel for analysis by both techniques, at different times of the disease. Samples of 22/49 patients (45%) presented circulating Sézary cells according to FCM: in 7/22 sample with lymphocytes CD4 T>5,000/uL; in 6/22 samples, ratio CD4/CD8>10; in 10/22, CD3dim o negative; in 16/22, CD7dim or negative; in 21/22 samples, CD26 was negative. In 27/49 (55%) peripheral involvement was detected by either or both methods, FCM or PCR. The degree of agreement was moderate (kappa = 0.457, p= 0.001). There were discrepancies in results in 13/49 (27%) patients (8 positive exclusively for FCM and 5 positive only for PCR). All patients but one with positive samples only by FCM presented CD4 T< 5,000/microL, ratio CD4/CD8< 10, and CD7 or CD26 negativity were between 35-60%. It is known that a subtype of central memory CD4 T cells shows similar phenotype (CD2dim, CD7dim or negative, CD3dim, CD26negative) and certain overlap between normal T cells and Sézary cells could exit. With a median follow-up of 16.5 (1-48) months after the study, there were significant differences in survival between the group of peripheral blood involvement B2 with respect to the other two groups, without involvement (B0) or with low tumor burden (B1) (p= 0.039) (Figure 1). No significant differences were found in survival between stage B0 and stage B1.
Conclusion
FCM is a useful tool for detection of low load of circulating Sézary cells, being CD26 negative the more sensitive marker to discriminate them. Because its phenotyping overlaps with normal cells, false positive could be discarded with an additional assay, such as PCR. The detection of a minimal expression in peripheral blood (<1,000 cells/microL) does not seem to impact the survival of these patients in our series.
(1) Olsen. Blood. 2007
(2) Scarisbrick. Eur J Cancer. 2018
Session topic: 19. Non-Hodgkin lymphoma Biology & Translational Research
Keyword(s): Cutaneous T-cell lymphoma, flow cytometry
Abstract: PB2325
Type: Publication Only
Background
Mycosis fungoides (MF) is the most frequent cutaneous T-cell lymphoma, generally with indolent course. It is accepted as a marker of poor prognosis a tumor load in peripheral blood of more than 1,000 cells/microL or more than 20% of the lymphocytes (B2), and equivalent to the nodal involvement (1). Less evidence exists on whether the detection of a lower tumor load in peripheral blood (B1<1,000 cel/microL) may have prognostic implications. In these cases of low tumor load, the manual Sézary count is difficult and requires a high experience. Techniques such as flow cytometry (FCM) and PCR (TCR gamma rearrangement) can help us to detect the presence of peripheral blood disease. Recently, the EORTC lymphoma cutaneous task force has recommended the FCM definition of blood-class even in patch/plaque/tumor MF (2), although the clinical implication of this minor blood involvement is to be defined.
Aims
To assess the degree of agreement between the results in the detection of Sézary cells in peripheral blood between FCM and PCR and the prognostic significance of the detection of low tumor load (stage B1).
Methods
The results obtained from 49 patients diagnosed of MF were retrospectively studied. The presence of circulating Sézary cells was analyzed by two techniques. For FCM, according to EORTC recommendations (1), presence of circulating Sézary cells was considered when the CD4/CD8 ratio was ≥10, or when the percentage of CD4 T negative for CD7>40% and/or for CD26>30%. The TCR gamma rearrangement was performed in parallel to identify clonal T cells. In addition, staging and survival data were collected.
Results
We identified 49 patients with MF, a median age 62 (19-88) years and 60% males. Samples of peripheral blood were sent in parallel for analysis by both techniques, at different times of the disease. Samples of 22/49 patients (45%) presented circulating Sézary cells according to FCM: in 7/22 sample with lymphocytes CD4 T>5,000/uL; in 6/22 samples, ratio CD4/CD8>10; in 10/22, CD3dim o negative; in 16/22, CD7dim or negative; in 21/22 samples, CD26 was negative. In 27/49 (55%) peripheral involvement was detected by either or both methods, FCM or PCR. The degree of agreement was moderate (kappa = 0.457, p= 0.001). There were discrepancies in results in 13/49 (27%) patients (8 positive exclusively for FCM and 5 positive only for PCR). All patients but one with positive samples only by FCM presented CD4 T< 5,000/microL, ratio CD4/CD8< 10, and CD7 or CD26 negativity were between 35-60%. It is known that a subtype of central memory CD4 T cells shows similar phenotype (CD2dim, CD7dim or negative, CD3dim, CD26negative) and certain overlap between normal T cells and Sézary cells could exit. With a median follow-up of 16.5 (1-48) months after the study, there were significant differences in survival between the group of peripheral blood involvement B2 with respect to the other two groups, without involvement (B0) or with low tumor burden (B1) (p= 0.039) (Figure 1). No significant differences were found in survival between stage B0 and stage B1.

Conclusion
FCM is a useful tool for detection of low load of circulating Sézary cells, being CD26 negative the more sensitive marker to discriminate them. Because its phenotyping overlaps with normal cells, false positive could be discarded with an additional assay, such as PCR. The detection of a minimal expression in peripheral blood (<1,000 cells/microL) does not seem to impact the survival of these patients in our series.
(1) Olsen. Blood. 2007
(2) Scarisbrick. Eur J Cancer. 2018
Session topic: 19. Non-Hodgkin lymphoma Biology & Translational Research
Keyword(s): Cutaneous T-cell lymphoma, flow cytometry


